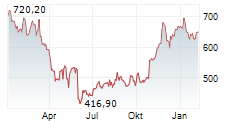
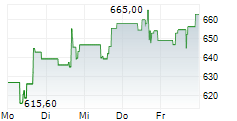
WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals Inc. (REGN) announced encouraging new data for its investigational gene therapy, DB-OTO, aimed at treating profound genetic hearing loss caused by variants in the otoferlin (OTOF) gene. The findings were published in The New England Journal of Medicine and presented at the annual meeting of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNSF).
The latest results from the pivotal CHORD trial revealed that 11 out of 12 participants experienced clinically meaningful improvements in hearing, with three achieving normal hearing levels. Among eight participants who had longer follow-up periods, all showed either continued improvement or stable hearing outcomes. Additionally, three individuals who completed speech assessments demonstrated significant gains in speech capabilities.
Regeneron said it plans to submit a U.S. regulatory application for DB-OTO later this year, pending discussions with the Food and Drug Administration (FDA). The therapy has already received multiple designations from the FDA, including Orphan Drug, Rare Pediatric Disease, Fast Track, and Regenerative Medicine Advanced Therapy status. The European Medicines Agency has also granted DB-OTO Orphan Drug Designation.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News