FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD) on Tuesday announced that Health Canada has approved Lyvdelzi for the treatment of adults with primary biliary cholangitis (PBC), a rare, chronic autoimmune liver disease that gradually destroys the liver's bile ducts.
Lyvdelzi is approved for use in combination with ursodeoxycholic acid (UDCA) in patients with an inadequate response to UDCA alone, or as monotherapy in patients unable to tolerate UDCA.
The market authorization has been granted with conditions, pending the results of clinical trials to confirm its therapeutic benefit. The conditional approval was primarily based on positive data from the Phase 3 RESPONSE study.
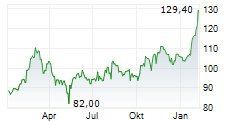
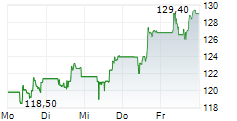
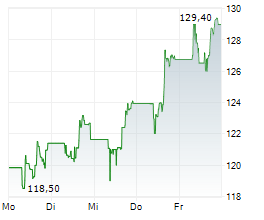
Gilead stock was down 0.32% in pre-market at $122.71.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News