FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD), Friday announced new long-term data, supporting Livdelzi's consistent efficacy and safety outcomes in patients with primary biliary cholangitis, a chronic, autoimmune disease of the bile ducts.
The real-world data analyzed 396 patients. Of these total patients, 130 switched from obeticholic acid and 266 used Livdelzi as second-line or monotherapy.
Presented at The Liver Meeting, the findings observed reductions in alkaline phosphatase or ALP in both groups, with most patients achieving ALP levels below 1.67×ULN.
Moreover, safety labs remained stable, and 93 percent of patients continued Livdelzi treatment throughout the observation period.
These data underscore Livdelzi's potential as an effective and well-tolerated alternative for patients switching from obeticholic acid, and as a second-line therapy.
Meanwhile, new interim results from the open-label Phase 3 ASSURE trial show that 67 percent of participants with PBC achieved a composite biochemical response, while 34 percent reached normalized ALP levels after three years of treatment with Livdelzi.
Further, new data from the pivotal RESPONSE study and its open-label extension, ASSURE, showed that Livdelzi delivers sustained and meaningful reduction in chronic itch.
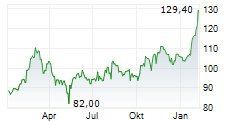
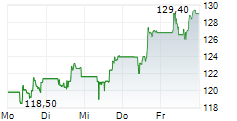
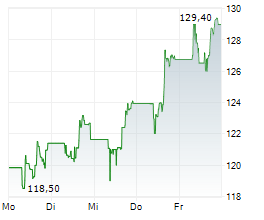
In the pre-market hours, GILD is trading at $124.30, up 0.73 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News