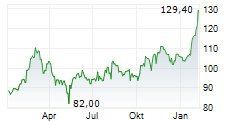
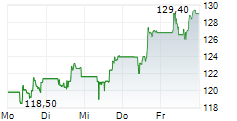
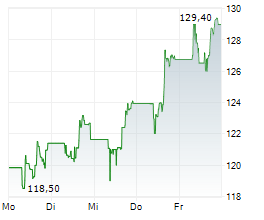
FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD) on Friday reported that the Phase 3 ASCENT-07 study, evaluating Trodelvy versus chemotherapy as a first-line treatment after endocrine therapy in HR+/HER2-negative metastatic breast cancer, did not meet its primary endpoint of progression-free survival (PFS).
Trodelvy is currently being studied in multiple Phase 3 trials across various tumor types.
The drug is approved in more than 50 countries for second-line or later treatment of metastatic triple-negative breast cancer (TNBC) and in over 40 countries for certain patients with pre-treated HR+/HER2-negative metastatic breast cancer.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News