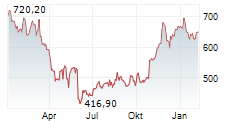
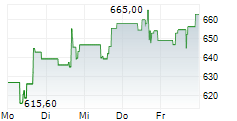
WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals, Inc. (REGN) announced that the European Commission has approved the PD-1 inhibitor Libtayo, or cemiplimab, as an adjuvant treatment for adult patients with cutaneous squamous cell carcinoma at high risk of recurrence after surgery and radiation. This expands the existing EU indication for Libtayo in advanced CSCC to include patients at high risk of disease recurrence.
Libtayo is also currently approved in the EU for the treatment of certain patients with advanced CSCC, advanced basal cell carcinoma, advanced non-small cell lung cancer and recurrent or metastatic cervical cancer.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News