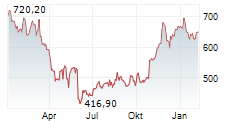
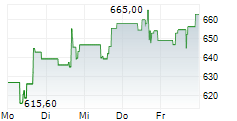
WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals (REGN) and Tessera Therapeutics announced a collaboration to develop and commercialize TSRA-196, Tessera's lead investigational in vivo Gene Writing program for the treatment of alpha-1 antitrypsin deficiency. Tessera expects to file an Investigational New Drug and multiple Clinical Trial Applications for TSRA-196 with the FDA by the end of the year.
The companies will share worldwide development costs and potential future profits relating to TSRA-196 equally. Tessera will receive $150 million, inclusive of a cash upfront payment and equity investment from Regeneron. Tessera is eligible to receive additional near and mid-term development milestone payments totaling $125 million. Tessera will lead the initial first-in-human trial. Regeneron will lead subsequent global development and commercialization.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News