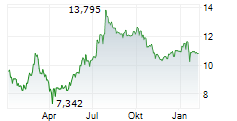
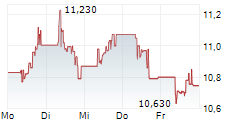
MADRID (dpa-AFX) - Grifols, S.A. (GRFS), a provider of plasma-derived medicines, said on Tuesday that its entire plasma value chain at Grifols Egypt for Plasma Derivatives (GEPD) has received certification from the European Medicines Agency (EMA), confirming compliance with Europe's highest standards for quality, safety and regulatory control.
GEPD, a joint venture between Egypt's National Service Projects Organization and Grifols, makes Egypt the first country in Africa and the Middle East to operate a fully integrated plasma collection and processing system, covering donor assessment, donation, testing, plasma processing and supply of plasma-derived medicines.
Plasma-derived medicines, essential for treating serious conditions, largely depend on U.S. supply, with Europe importing nearly 40% of its plasma. EMA certification of Grifols Egypt positions the country as a potential hub to help strengthen Europe's plasma supply security.
Tomas Daga, Vice President of Grifols Egypt, said, 'This certification confirms the quality and rigor of the work carried out by the teams over these five years to create Africa's first integrated plasma platform and align it with the most demanding international standards.'
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News