WASHINGTON (dpa-AFX) - Moderna, Inc. (MRNA), a biotechnology company, Friday reported narrower loss for the fourth quarter compared to the same period last year.
Quarterly loss narrowed to $826 million or $2.11 per share from $1120 million or $2.91 per share of last year.
Loss from operations reduced to $857 million from $1246 million of the previous year.
Revenue decreased to $678 million from $966 million of the prior year.
However, this was on the higher end of the Company's prior expectations, and was driven primarily by COVID vaccine sales.
For the Seasonal flu and COVID vaccine the company's mRNA-1083 regulatory filing is under review in Europe and Canada and is awaiting further guidance from U.S. FDA on refiling the submission for its flu/COVID combination vaccine.
For the seasonal flu vaccine, the Company's mRNA-1010 regulatory filings are under review in Europe, Canada and Australia and potential approvals are expected to begin in 2026, whereas it has received a Refusal-to-File letter from the U.S. FDA and has requested a Type A meeting to understand the path forward.
For the Norovirus vaccine, Moderna's ongoing Phase 3 safety and efficacy study of mRNA-1403 is fully enrolled in a second Northern Hemisphere season (2025-2026) with a data readout expected in 2026, subject to case accruals.
For its Intismeran autogene, the Company is advancing mRNA-4157 in collaboration with Merck, with eight total Phase 2 and Phase 3 clinical trials underway across multiple tumor types.
For mRNA-4359, Moderna's Phase 1/2 study of mRNA-4359, an investigational wholly-owned cancer antigen therapy, is ongoing.
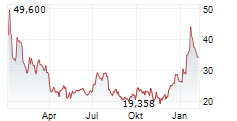
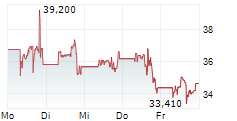
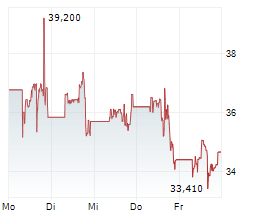
In pre-market activity, MRNA shares were trading at $41, up 2.22% on the Nasdaq.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News