WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals, Inc. (REGN) announced Thursday that the U.S. Food and Drug Administration (FDA) has accepted for Priority Review the Biologics License Application (BLA) for garetosmab for the treatment of adults with fibrodysplasia ossificans progressiva (FOP). The target action date for the FDA decision is August 2026.
Garetosmab is a monoclonal antibody that blocks Activin A, a protein that Regeneron scientists discovered to be critical in the development of heterotopic ossification (HO) lesions in people with FOP.
The BLA is supported by efficacy and safety data from the positive Phase 3 OPTIMA trial evaluating garetosmab in adults with FOP. Both garetosmab doses (3 mg/kg and 10 mg/kg) evaluated in the trial were highly efficacious in reducing the total number and volume of new HO lesions at 56 weeks, compared to placebo.
Regarding the primary endpoint analysis of reduction in total number of new HO lesions compared to placebo, those receiving the 3 mg/kg dose experienced a 94% reduction. Meanwhile, those receiving the 10 mg/kg dose experienced a 90% reduction.
A post-hoc analysis also found both doses of garetosmab demonstrated a greater than 99% reduction in mean total volume (cm3) of new HO lesions compared to placebo.
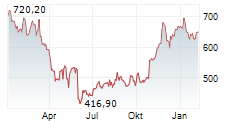
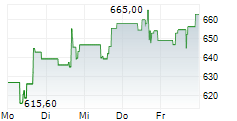
In Thursday's pre-market trading, REGN is trading on the Nasdaq at $789.00, down $3.16 or 0.40 percent.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News