| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 4,820 | 4,940 | 07:31 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 23.02. | ASCENTAGE PHARMA GROUP INTERNATIONAL - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 12.02. | Rodman & Renshaw Initiates Ascentage Pharma Group International (AAPG) with Buy Rating, $48 Target | 2 | Insider Monkey | ||
| 06.02. | ASCENTAGE PHARMA GROUP INTERNATIONAL - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 06.02. | ASCENTAGE PHARMA GROUP INTERNATIONAL: Ascentage Pharma Announces IND Clearance by the China CDE for BTK Degrader APG-3288 | 271 | GlobeNewswire (Europe) | ROCKVILLE, Md. and SUZHOU, China, Feb. 05, 2026 (GLOBE NEWSWIRE) -- Ascentage Pharma Group International (NASDAQ: AAPG; HKEX: 6855), a global, commercial stage, integrated biopharmaceutical company... ► Artikel lesen | |
| 15.01. | ASCENTAGE PHARMA GROUP INTERNATIONAL - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 13.01. | Rodman & Renshaw initiates coverage on Ascentage Pharma stock with Buy rating | 3 | Investing.com | ||
| ASCENTAGE PHARMA GROUP Aktie jetzt für 0€ handeln | |||||
| 07.01. | ASCENTAGE PHARMA GROUP INTERNATIONAL - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 07.01. | ASCENTAGE PHARMA GROUP INTERNATIONAL: Ascentage Pharma Announces IND Clearance by the U.S. Food and Drug Administration for BTK Degrader APG-3288 | 308 | GlobeNewswire (Europe) | Novel next-generation Bruton tyrosine kinase (BTK)-targeted protein degrader APG-3288 has received investigational new drug (IND) clearance from the U.S. FDA, marking another major expansion to the... ► Artikel lesen | |
| 31.12.25 | ASCENTAGE PHARMA GROUP INTERNATIONAL - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 30.12.25 | ASCENTAGE-B (06855): LIST OF DIRECTORS AND THEIR ROLES AND FUNCTIONS | 1 | HKEx | ||
| 30.12.25 | ASCENTAGE-B (06855): (1) CHANGE IN COMPOSITION OF NOMINATION COMMITTEE; (2) APPOINTMENT OF LEAD INDEPENDENT NON-EXECUTIVE DIRECTOR; (3) ESTABLISHMENT ... | 2 | HKEx | ||
| 17.12.25 | ASCENTAGE PHARMA GROUP INTERNATIONAL - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 09.12.25 | ASCENTAGE PHARMA GROUP INTERNATIONAL: ASH 2025 | Updated Data for Ascentage Pharma's Olverembatinib in Second-Line CML-CP Showing Encouraging Potential for Early-Line Treatment | 1 | GlobeNewswire (USA) | ||
| 09.12.25 | ASCENTAGE PHARMA GROUP INTERNATIONAL: ASH 2025 | Ascentage Pharma Presents First Dataset from Phase III POLARIS-1 Study of Olverembatinib in Newly Diagnosed Ph+ ALL Shows a Best MRD-Negativity CR Rate Exceeding 60% | 2 | GlobeNewswire (USA) | ||
| 09.12.25 | ASCENTAGE PHARMA GROUP INTERNATIONAL: ASH 2025 | Ascentage Pharma Presents Four-Year Follow-Up Data from Registrational Phase II Study of Olverembatinib, Reaffirming Differentiated Long-Term Efficacy and Safety in TKI-Resistant/Intolerant CML-CP | 1 | GlobeNewswire (USA) | ||
| 08.12.25 | ASCENTAGE PHARMA GROUP INTERNATIONAL: ASH 2025 | Ascentage Pharma Presents Encouraging Data from Phase Ib/II Study of Bcl-2 Inhibitor Lisaftoclax in Venetoclax-Exposed Patients with Myeloid Malignances | 1 | GlobeNewswire (USA) | ||
| 06.12.25 | ASCENTAGE PHARMA GROUP INTERNATIONAL: Ascentage Pharma Presents Pivotal China Registrational Study Data for Lisaftoclax in Oral Report at 2025 American Society of Hematology (ASH) Annual Meeting | 124 | GlobeNewswire (Europe) | Lisaftoclax monotherapy demonstrated significant and durable clinical efficacy and a manageable safety profile in patients with heavily pretreated BTK-refractory R/R CLL/SLL, underscoring its utility... ► Artikel lesen | |
| 05.12.25 | ASCENTAGE PHARMA GROUP INTERNATIONAL - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 05.12.25 | Ascentage Gets Regulatory Clearance In The US And Europe To Conduct POLARIS-1 Trial | 2 | RTTNews | ||
| 05.12.25 | Ascentage Pharma To Launch Global Phase III Study Of Olverembatinib In Ph+ ALL | 316 | AFX News | BEIJING (dpa-AFX) - Ascentage Pharma Group International (AAPG, 6855.HK) announced that it received clearance from both the US Food and Drug Administration (FDA) and the European Medicines Agency... ► Artikel lesen |
1 2 3 Weiter >>
46 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 41,960 | 0,00 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| EVOTEC | 5,880 | 0,00 % | Evotec: Das Biotech-Drama geht in die nächste Runde | Die Aktie des Hamburger Wirkstoffforschers Evotec versucht erneut den Befreiungsschlag, doch das Misstrauen am Markt ist greifbar. Nach einer beispiellosen Serie von Rückschlägen - von Management-Skandalen... ► Artikel lesen | |
| BIONTECH | 93,10 | -0,21 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | -79,74 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | +24,33 % | Cardio Diagnostics Holdings, Inc. - 8-K, Current Report | ||
| ADMA BIOLOGICS | 15,580 | +2,70 % | ADMA Q4 EPS Up 14% Y/Y, Revenues Gain From Strong Asceniv Performance | ||
| RELAY THERAPEUTICS | 10,250 | +12,02 % | Relay Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| PRAXIS PRECISION MEDICINES | 336,75 | -1,05 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,820 | -3,48 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| KYMERA THERAPEUTICS | 91,36 | -3,85 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| COGENT BIOSCIENCES | 38,870 | -1,55 % | Cogent Biosciences, Inc.: Cogent Biosciences Highlights Additional Data with Six Bezuclastinib Posters from SUMMIT Trial at 2026 AAAAI Annual Meeting | ||
| EDGEWISE THERAPEUTICS | 30,450 | +2,77 % | Edgewise Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results with Strong Progress Across Muscular Dystrophy and Cardiovascular Programs | - CIRRUS-HCM 12-week data of EDG-7500 in obstructive and nonobstructive hypertrophic cardiomyopathy (HCM) expected in H1 2026 -
- Phase 1 healthy adult trial... ► Artikel lesen |
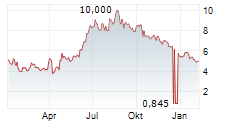
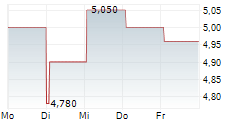
ASCENTAGE PHARMA GROUP INTERNATIONAL gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 4,580 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ASCENTAGE PHARMA GROUP INTERNATIONAL finden Sie auf Wallstreet Online.