- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| BIOMX Aktie jetzt für 0€ handeln | |||||
| Mi | BiomX Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 19.02. | BiomX Inc. - 10-K, Annual Report | 5 | SEC Filings | ||
| 10.02. | BiomX Inc. - 8-K, Current Report | 10 | SEC Filings | ||
| 27.01. | BiomX Inc: BiomX Issues Statement Regarding Recent Common Stock Trading Activity | 159 | GlobeNewswire (Europe) | NESS ZIONA, Israel, Jan. 27, 2026 (GLOBE NEWSWIRE) -- BiomX Inc. (NYSE American: PHGE) ("BiomX" or the "Company"), a clinical-stage company advancing novel natural and engineered phage therapies targeting... ► Artikel lesen | |
| 27.01. | Morning Market Movers: Nuwellis, BiomX, Brand Engagement Network, Blaize Holdings See Big Swings | 416 | AFX News | WOONSOCKET (dpa-AFX) - At 7:02 a.m. ET on Tuesday, premarket trading is seeing notable activity in several stocks, with early price movements signaling potential opportunities before the opening... ► Artikel lesen | |
| 14.01. | BiomX Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 07.01. | BiomX Inc. - 8-K, Current Report | - | SEC Filings | ||
| 29.12.25 | BiomX Inc. - 8-K, Current Report | 8 | SEC Filings | ||
| 29.12.25 | BiomX Inc: BiomX Announces $3.0 Million Private Placement | 3 | GlobeNewswire (USA) | ||
| 17.12.25 | BiomX collapses into court proceedings after clinical trial fails | 6 | CTech | ||
| 08.12.25 | BiomX Inc: BiomX Announces Discontinuation of Phase 2b BX004 Trial Following Internal Review | 203 | GlobeNewswire (Europe) | Following internal analysis and Data Monitoring Committee (DMC) feedback, the Company has elected to discontinue the BX004 Cystic Fibrosis (CF) Phase 2b trial BiomX continues to see potential in... ► Artikel lesen | |
| 08.12.25 | BiomX Inc. - 8-K, Current Report | - | SEC Filings | ||
| 28.11.25 | H.C. Wainwright reiterates Buy rating on BiomX stock amid FDA queries | 10 | Investing.com | ||
| 28.11.25 | BiomX: H.C. Wainwright bestätigt Kaufempfehlung trotz FDA-Rückfragen zum Inhalator | 5 | Investing.com Deutsch | ||
| 26.11.25 | BiomX Awaits FDA Clearance To Restart Cystic Fibrosis Trial After Nebulizer Device Review | 8 | Benzinga.com | ||
| 26.11.25 | BiomX To Continue Cystic Fibrosis Study With Revised Dosing Of BX004; Data Expected In Q2, 2026 | 1 | RTTNews | ||
| 25.11.25 | BiomX Inc: BiomX Provides Update on BX004 Phase 2b Trial in Cystic Fibrosis | 259 | GlobeNewswire (Europe) | The Company continues working with the third-party manufacturer to address recent FDA follow-up information requests which are required to lift the clinical hold concerning the nebulizer device used... ► Artikel lesen | |
| 25.11.25 | BiomX Inc. - 8-K, Current Report | - | SEC Filings | ||
| 14.11.25 | BiomX announces 1-for-19 reverse stock split | 4 | Seeking Alpha | ||
| 14.11.25 | BiomX to implement 1-for-19 reverse stock split on November 25 | 1 | Investing.com |
1 2 Weiter >>
38 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,60 | +0,32 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| AMGEN | 330,20 | +0,50 % | Is Amgen Stock Outperforming the Dow? | ||
| INOVIO PHARMACEUTICALS | 1,500 | -2,60 % | Is Inovio Pharmaceuticals Stock Going to $0? | ||
| DEFENCE THERAPEUTICS | 0,371 | -1,85 % | Defence Therapeutics Inc.: Defence Therapeutics to Showcase Accum Platform at Key International Industry Events in March | Montreal, Quebec--(Newsfile Corp. - March 2, 2026) - Defence Therapeutics Inc. (CSE: DTC) (FSE: DTC) (OTCQB: DTCFF) ("Defence" or the "Company"), a publicly traded biotechnology company advancing... ► Artikel lesen | |
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,360 | -4,61 % | PACIFIC BIOSCIENCES OF CALIFORNIA, INC. - S-8, Securities to be offered to employees in employee benefit plans | ||
| CARDIOL THERAPEUTICS | 0,874 | +0,81 % | Cardiol Therapeutics Inc.: Cardiol Therapeutics to Present at TD Cowen 46th Annual Health Care Conference | Toronto, Ontario--(Newsfile Corp. - February 25, 2026) - Cardiol Therapeutics Inc. (NASDAQ: CRDL) (TSX: CRDL) ("Cardiol" or the "Company"), a late-stage life sciences company focused on advancing... ► Artikel lesen | |
| REDHILL BIOPHARMA | 0,970 | -1,02 % | RedHill Biopharma Ltd. - 6-K, Report of foreign issuer | ||
| KYNTRA BIO | 5,850 | 0,00 % | Kyntra Bio Announces Positive Data from the Investigator-Sponsored Phase 1b/2 Study of FG-3246 in Combination with Enzalutamide in Patients with Metastatic Castration Resistant Prostate Cancer to Be Presented at ASCO GU 2026 | FG-3246 and enzalutamide combination therapy, in biomarker unselected patients with androgen receptor pathway inhibitor (ARPI)-treated, taxane-naïve metastatic castration-resistant prostate cancer... ► Artikel lesen | |
| LEXAGENE | - | - | SOLARIA ENERGIA Y MEDIOAMBIENTE, S.A.: Solaria reports it has signed an agreement with Stoneshield Capital to create a new joint venture, Gravyx, specialising in standalone batteries. | ||
| ARMATA PHARMACEUTICALS | 9,300 | +2,20 % | Armata Pharmaceuticals, Inc.: Armata Pharmaceuticals Receives FDA Qualified Infectious Disease Product (QIDP) Designation for AP-SA02 | Intravenous use as a QIDP for adjunct treatment of complicated bacteremia caused by Staphylococcus aureus
QIDP Designation provides for five years of market exclusivity... ► Artikel lesen | |
| VAXCYTE | 52,00 | -0,95 % | Vaxcyte, Inc.: Vaxcyte Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | Comprehensive VAX-31 Adult Phase 3 Clinical Program, Finalized in Consultation and Alignment with FDA, Advances with Three Phase 3 Studies Underway to Support Planned BLA Submission Topline Safety... ► Artikel lesen | |
| VERRICA PHARMACEUTICALS | 4,740 | +1,72 % | Verrica Pharmaceuticals Inc.: Verrica Pharmaceuticals Appoints Chris Chapman as Chief Commercial Officer | WEST CHESTER, Pa., Feb. 12, 2026 (GLOBE NEWSWIRE) -- Verrica Pharmaceuticals Inc. ("Verrica") (Nasdaq: VRCA), a dermatology therapeutics company developing and selling medications for skin diseases... ► Artikel lesen | |
| HOOKIPA PHARMA | 0,870 | +3,55 % | HOOKIPA Pharma Inc.: HOOKIPA Pharma Announces Sale of Oncology Assets to NeoTrail Therapeutics | NEW YORK and VIENNA, Austria, Feb. 03, 2026 (GLOBE NEWSWIRE) -- HOOKIPA Pharma Inc. (OTCID: HOOK, "HOOKIPA", the "Company") today announced the sale of its immuno-oncology related assets, consisting... ► Artikel lesen | |
| TAYSHA GENE THERAPIES | 4,580 | +0,99 % | Taysha Gene Therapies, Inc.: Taysha Gene Therapies Announces Progress Across TSHA-102 Pivotal Gene Therapy Program in Rett Syndrome | First patient dosed in REVEAL pivotal trial evaluating TSHA-102 (N=15, aged 6 to Reached written alignment with FDA on inclusion of =3 months of safety data from ASPIRE trial evaluating TSHA-102... ► Artikel lesen | |
| QIAGEN | 41,295 | -0,23 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen |
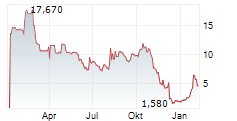
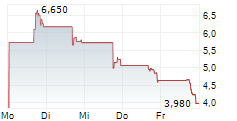
Das Unternehmen BIOMX INC kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 4,590 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BIOMX INC finden Sie auf Wallstreet Online.