- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 23.02. | Fortress Biotech, Inc.: Fortress Biotech's Subsidiary Cyprium Therapeutics Enters into Agreement to Sell Rare Pediatric Disease Priority Review Voucher for $205 Million | 198 | GlobeNewswire (Europe) | MIAMI, Feb. 23, 2026 (GLOBE NEWSWIRE) -- Fortress Biotech, Inc. (Nasdaq: FBIO) ("Fortress") and its majority-owned subsidiary, Cyprium Therapeutics, Inc. ("Cyprium"), today announced that Cyprium... ► Artikel lesen | |
| 23.02. | Fortress Biotech, Inc. - 8-K, Current Report | 5 | SEC Filings | ||
| 14.01. | Fortress Biotech receives FDA approval for Zycubo to treat Menkes disease | 13 | Pharmaceutical Technology | ||
| 13.01. | Fortress Bio gains on FDA approval of copper replacement therapy | 6 | Seeking Alpha | ||
| FORTRESS BIOTECH INC PREF Aktie jetzt für 0€ handeln | |||||
| 13.01. | Fortress Biotech, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 13.01. | Fortress Biotech, Inc.: Fortress Biotech and Cyprium Therapeutics Announce U.S. FDA Approval of ZYCUBO (copper histidinate), the First and Only Approved Treatment for Menkes Disease in the United States | 772 | GlobeNewswire (Europe) | Rare Pediatric Disease Priority Review Voucher (PRV) granted by FDA at approval to be transferred from Sentynl Therapeutics to Cyprium Cyprium eligible to receive tiered royalties and up to $129 million... ► Artikel lesen | |
| 15.12.25 | FDA sets new PDUFA date for Fortress Biotech's Menkes disease therapy | 31 | Investing.com | ||
| 15.12.25 | Fortress Biotech, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 14.11.25 | Fortress Biotech, Inc.: Fortress Biotech Reports Third Quarter 2025 Financial Results and Recent Corporate Highlights | 644 | GlobeNewswire (Europe) | Total net revenue increased 20.5% to $17.6 million for third quarter of 2025 compared to the third quarter of 2024 Fortress subsidiary Checkpoint Therapeutics acquired by Sun Pharma; Fortress received... ► Artikel lesen | |
| 14.11.25 | Fortress Biotech, Inc. - 10-Q, Quarterly Report | 3 | SEC Filings | ||
| 14.11.25 | Fortress Biotech, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 11.11.25 | Fortress Biotech Q3 Earnings Preview | 5 | Seeking Alpha | ||
| 21.10.25 | Fortress Biotech: Aktie legt zu - Gichtmittel der Tochtergesellschaft erreicht Phase-3-Studie | 16 | Investing.com Deutsch | ||
| 21.10.25 | Fortress Biotech stock rises after subsidiary's gout treatment enters Phase 3 trials | 3 | Investing.com | ||
| 21.10.25 | Fortress Biotech, Inc.: Fortress Biotech and Subsidiary Urica Therapeutics Announce First Patients Dosed in Crystalys Therapeutics' Global Phase 3 Trials of Dotinurad for the Treatment of Gout | 274 | GlobeNewswire (Europe) | MIAMI, Oct. 21, 2025 (GLOBE NEWSWIRE) -- Urica Therapeutics, Inc. ("Urica" or the "Company"), a Fortress Biotech, Inc. (Nasdaq: FBIO) ("Fortress") subsidiary, today announced that Crystalys Therapeutics... ► Artikel lesen | |
| 02.10.25 | FDA verweigert Zulassung für Medikament von Fortress Biotech wegen Produktionsmängeln | 20 | Investing.com Deutsch | ||
| 02.10.25 | Fortress Biotech stock faces setback as FDA issues CRL for CUTX-101 | 3 | Investing.com | ||
| 01.10.25 | Fortress Biotech stock plummets after FDA rejects Menkes disease drug | 13 | Investing.com | ||
| 01.10.25 | Fortress Biotech plunges as FDA rejects rare disease drug | 20 | Seeking Alpha | ||
| 01.10.25 | Fortress Biotech, Inc. - 8-K, Current Report | 5 | SEC Filings |
1 2 Weiter >>
27 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| STRYKER | 332,10 | +1,25 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| REGENERON PHARMACEUTICALS | 672,60 | +1,66 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| VIKING THERAPEUTICS | 28,920 | +0,84 % | Viking Therapeutics: The High-Stakes Weight Loss Contender | ||
| INTELLIA THERAPEUTICS | 13,000 | +11,49 % | Intellia Therapeutics, Inc.: Intellia Therapeutics Announces FDA Lift of Clinical Hold on MAGNITUDE Phase 3 Clinical Trial in ATTR-CM | CAMBRIDGE, Mass., March 02, 2026 (GLOBE NEWSWIRE) -- Intellia Therapeutics, Inc. (Nasdaq: NTLA), a leading biopharmaceutical company focused on revolutionizing medicine leveraging CRISPR gene editing... ► Artikel lesen | |
| TEMPUS AI | 44,600 | -1,33 % | Tempus AI Quartalszahlen: 90 % Umsatzplus bestätigen KI-Trend in der Medizin | CHICAGO, Illinois (IT-Times) - Das Diagnose-Technologie-Unternehmen Tempus AI hat seine Ergebnisse für das vierte Quartal 2025 veröffentlicht und ein rasantes Umsatzwachstum verzeichnet. Tempus AI -... ► Artikel lesen | |
| NUREXONE BIOLOGIC | 0,415 | -2,35 % | NurExone Biologic: Pionier in der Exosomen-Medizin! | ||
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,360 | -4,61 % | PACIFIC BIOSCIENCES OF CALIFORNIA, INC. - S-8, Securities to be offered to employees in employee benefit plans | ||
| IMMUNITYBIO | 9,098 | +9,75 % | ImmunityBio, Inc.: ImmunityBio Reports 700% Year-Over-Year Revenue Growth, Expanded ANKTIVA Approvals in Lung Cancer and Global Commercial Partnerships in 33 Countries with Label Expansion Plans Globally | 2025 Sales Momentum: ANKTIVA net product revenue increased 20% quarter-over-quarter, with full-year net product revenue of $113 million, representing an approximately 700% increase year-over-year... ► Artikel lesen | |
| RECURSION PHARMACEUTICALS | 3,605 | -2,04 % | Recursion Pharmaceuticals: Recursion Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | Delivered first clinical validation of the Recursion full stack AI Operating System in FAP, demonstrating translation from AI-driven biological insight to meaningful patient outcomes; multiple clinical... ► Artikel lesen | |
| GINKGO BIOWORKS | 5,600 | -1,75 % | Ginkgo Bioworks Reports Fourth Quarter and Full Year 2025 Financial Results, Announces Focus on Autonomous Labs Offerings and Divestiture of its Non-Core Biosecurity Business | Ginkgo provides an update on its fourth quarter financial results
BOSTON, Feb. 26, 2026 /PRNewswire/ -- Ginkgo Bioworks Holdings, Inc. (NYSE: DNA, "Ginkgo") today... ► Artikel lesen | |
| IOVANCE BIOTHERAPEUTICS | 3,232 | -1,72 % | Iovance Biotherapeutics, Inc.: Iovance Announces Positive Results from the First Clinical Trial for TIL Cell Therapy in Soft Tissue Sarcomas | 50% Objective Response Rate (ORR) in Advanced Sarcomas Significant Market Opportunity with More than 8,000 Patients Diagnosed Annually in the U.S. and Europe SAN CARLOS, Calif., Feb. 24, 2026 (GLOBE... ► Artikel lesen | |
| CYTOMX THERAPEUTICS | 4,622 | +2,89 % | SD-Tipp CytomX: Was tun nach +1.438% in 10 Monaten? | Unsere Aktien-Empfehlung CytomX Therapeutics erlebt derzeit einen außergewöhnlich starken Kursverlauf. Innerhalb kurzer Zeit hat sich das Papier von einem lange übersehenen Small-Cap-Titel zu einem... ► Artikel lesen | |
| SANA BIOTECHNOLOGY | 4,120 | -2,14 % | Sana Biotechnology (SANA) Appoints Industry Veteran Brian Piper as New CFO | ||
| TERNS PHARMACEUTICALS | 36,200 | +1,12 % | Terns Pharmaceuticals, Inc.: Terns Announces Closing of Public Offering of Common Stock, Including Full Exercise of Underwriters' Option to Purchase Additional Shares | FOSTER CITY, Calif., Dec. 11, 2025 (GLOBE NEWSWIRE) -- Terns Pharmaceuticals, Inc. ("Terns" or the "Company") (Nasdaq: TERN), a clinical-stage oncology company, today announced the closing of its... ► Artikel lesen | |
| BIOMEA FUSION | 1,355 | -0,73 % | Biomea Fusion, Inc. - 8-K, Current Report |
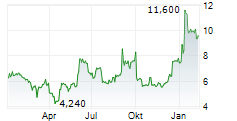
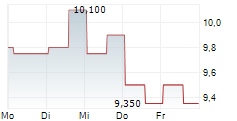
Das Unternehmen FORTRESS BIOTECH INC PREF kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +0,88 % bei einem Aktienkurs von 11,500 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu FORTRESS BIOTECH INC PREF finden Sie auf Wallstreet Online.