- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | GT Biopharma reports FY results | 1 | Seeking Alpha | ||
| Mo | GT Biopharma, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| Mo | GT Biopharma, Inc.: GT Biopharma Reports Full Year 2025 Financial Results | 785 | GlobeNewswire (Europe) | Phase 1 trial evaluating GTB-3650 TriKE- continues to actively enroll, with the next update anticipated in Q3 2026 following completion of enrollment in dose Cohort 5 Phase 1 basket trial evaluating... ► Artikel lesen | |
| Mo | GT Biopharma, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 03.02. | GT Biopharma, Inc.: GT Biopharma Announces FDA Clearance of Investigational New Drug (IND) Application for GTB-5550 TriKE, a B7-H3-Targeted Natural Killer (NK) Cell Engager for Solid Tumors Expressing B7-H3 | 176 | GlobeNewswire (Europe) | GTB-5550 Phase 1 dose escalation basket trial expected to initiate mid-2026 Phase 1 protocol allows multiple solid tumor types known to express B7-H3 Unaudited proforma cash balance as of January... ► Artikel lesen | |
| 21.01. | GT Biopharma, Inc. - S-1, General form for registration of securities | 2 | SEC Filings | ||
| 15.01. | GT Biopharma Submits IND For GTB-5550 TriKE To Treat Solid Tumors | 1 | RTTNews | ||
| 15.01. | GT Biopharma submits IND application for solid tumor cancer treatment | 1 | Investing.com | ||
| GT BIOPHARMA Aktie jetzt für 0€ handeln | |||||
| 15.01. | GT Biopharma, Inc.: GT Biopharma Announces IND Submission for GTB-5550 TriKE, a B7-H3-targeted natural killer (NK) cell engager for B7-H3 expressing solid tumor cancers | 229 | GlobeNewswire (Europe) | GT Biopharma targets a portion of the estimated $362 billion global solid tumor market Preliminary, unaudited cash balance of approximately $7 million as of December 31, 2025 anticipated to extend... ► Artikel lesen | |
| 15.01. | EXCLUSIVE: GT Biopharma Plans 2026 Trial For New Cancer Treatment | 2 | Benzinga.com | ||
| 26.11.25 | GT Biopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 19.11.25 | GT Biopharma-Aktie legt zu: Phase-1-Studie erreicht höhere Dosierungsstufe | 2 | Investing.com Deutsch | ||
| 19.11.25 | GT Biopharma, Inc. Offers Near-Term Catalyst Opportunity with Advancing Phase 1 Trials | 286 | ACCESS Newswire | NAPLES, FL / ACCESS Newswire / November 19, 2025 / Patients battling aggressive blood cancers face limited treatment options and uncertain outcomes. Scientists have increasingly turned to the body's... ► Artikel lesen | |
| 14.11.25 | GT Biopharma, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 14.11.25 | GT Biopharma reports Q3 results | 1 | Seeking Alpha | ||
| 14.11.25 | GT Biopharma, Inc.: GT Biopharma Reports Third Quarter 2025 Financial Results and Provides Corporate Update | 500 | GlobeNewswire (Europe) | Phase 1 trial evaluating GTB-3650 TriKE® for relapsed or refractory (r/r) CD33 expressing hematologic malignancies continues to actively enroll with the next update anticipated in Q1 2026 GTB-5550... ► Artikel lesen | |
| 14.11.25 | GT Biopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 24.10.25 | GT Biopharma files to sell 14.59M shares of common stock for holders | 1 | Seeking Alpha | ||
| 24.10.25 | GT Biopharma, Inc. - S-1, General form for registration of securities | 1 | SEC Filings | ||
| 23.10.25 | GT Biopharma advances to Cohort 4 in Phase 1 trial of cancer therapy | 2 | Investing.com |
1 2 Weiter >>
29 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| FRESENIUS | 48,180 | -0,06 % | Mehr Dividende, mehr Gewinn: Darum kann Fresenius nicht punkten | Die Fresenius Aktie steht zu Beginn des Handelstages unter Abgabedruck: Auch wenn die Pharmasparte Kabi und das Krankenhausgeschäft gut laufen und dem DAX-Konzern für 2025 zu soliden Ergebnissen verholfen... ► Artikel lesen | |
| FRESENIUS MEDICAL CARE | 39,150 | -0,91 % | Börse am Morgen: AMD, E.On, FMC, Indus, Nvidia, Anthropic - Nord/LB Marktbericht | Das Verbrauchervertrauen in den USA hat sich im Februar überraschend aufgehellt. Das Barometer für die Konsumlaune stieg um 2,2 Punkte auf 91,2 Zähler, wie das Conference Board mitteilte. Befragte Ökonomen... ► Artikel lesen | |
| SIEMENS HEALTHINEERS | 41,680 | +4,02 % | RBC stuft Siemens Healthineers auf 'Outperform' | NEW YORK (dpa-AFX Analyser) - Die kanadische Bank RBC hat Siemens Healthineers mit einem Kursziel von 55 Euro auf "Outperform" belassen. Die Nervosität der Anleger vor einem möglichen Verkauf des Diagnostikgeschäfts... ► Artikel lesen | |
| CARL ZEISS MEDITEC | 25,880 | +2,05 % | Carl Zeiss Meditec-Aktie: Überraschende Erholung - Was steckt dahinter? | ||
| ECKERT & ZIEGLER | 15,210 | +3,54 % | EQS-News: Eckert & Ziegler SE: Eckert & Ziegler kooperiert mit Molecular Partners bei der Entwicklung von Radio-DARPin-Therapeutika | EQS-News: Eckert & Ziegler SE
/ Schlagwort(e): Kooperation/Vereinbarung
Eckert & Ziegler kooperiert mit Molecular Partners bei der Entwicklung von Radio-DARPin-Therapeutika
26.02.2026... ► Artikel lesen | |
| UNITEDHEALTH | 250,80 | +0,74 % | UnitedHealth-Aktie: Erholung in Sicht - Was Anleger jetzt wissen müssen | ||
| GERRESHEIMER | 17,150 | +6,46 % | Shortseller-Positionen aktuell: Auto1, FMC, Gerresheimer, Hensoldt, Nagarro, TeamViewer, Zalando | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| DRAEGERWERK | 89,60 | +2,87 % | Draegerwerk AG & Co. KGaA: Zollentlastung bereits eingepreist, Aufwärtspotenzial wird kleiner, Kursziel erhöht; auf HALTEN gestuft. | Die Zollerlassungen in den USA bieten einen inkrementellen Unterstützungseffekt für die Draegerwerk, jedoch wird der Nutzen voraussichtlich schrittweise erfolgen. Während bestimmte Zölle aufgehoben... ► Artikel lesen | |
| MEDTRONIC | 82,34 | -1,12 % | Medtronic Launches MiniMed Go Smart MDI System In Europe | WASHINGTON (dpa-AFX) - Medtronic (MDT) announced the commercial launch in Europe of the MiniMed Go Smart MDI system with the Simplera sensor. The launch will be rolled out gradually across Europe... ► Artikel lesen | |
| INTUITIVE SURGICAL | 430,90 | +0,64 % | Intuitive Surgical: Steht ein weiterer Rücksetzer bevor? | Die Intuitive-Surgical-Aktie zeigt seit dem Jahreswechsel eine deutlich schwächere Struktur. Rutscht der Kurs in Richtung der nächsten Unterstützung ab Den vollständigen Artikel lesen ... ► Artikel lesen | |
| THERMO FISHER | 440,50 | +0,73 % | THERMO FISHER SCIENTIFIC INC. - 10-K, Annual Report | ||
| TELADOC HEALTH | 4,457 | +1,41 % | Teladoc Health Q4 Loss Narrows | WASHINGTON (dpa-AFX) - Teladoc Health, Inc. (TDOC) on Wednesday reported a fourth-quarter net loss of $25.1 million, or $0.14 per share, compared to $48.4 million or $0.28 per share last year.Fourth-quarter... ► Artikel lesen | |
| STRATEC | 20,000 | 0,00 % | EQS-PVR: STRATEC SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide distribution | EQS Voting Rights Announcement: STRATEC SE
STRATEC SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide... ► Artikel lesen | |
| BRIGHTSPRING HEALTH SERVICES | 41,960 | +3,78 % | BrightSpring Health Services, Inc. Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Full Year 2026 Guidance | LOUISVILLE, Ky., Feb. 27, 2026 (GLOBE NEWSWIRE) -- BrightSpring Health Services, Inc. ("BrightSpring" or the "Company") (NASDAQ: BTSG), a leading provider of home and community-based health services... ► Artikel lesen | |
| FIREFLY NEUROSCIENCE | 1,550 | +116,48 % | Firefly Neuroscience Skyrockets - Brain Scan Breakthrough Fuels Rally |
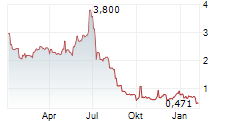
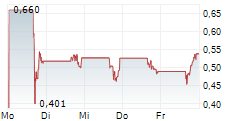
Das Unternehmen GT BIOPHARMA INC kann der Branche Gesundheitswesen zugeordnet werden. Die relative Kursveränderung beträgt aktuell -2,59 % bei einem Aktienkurs von 0,448 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GT BIOPHARMA INC finden Sie auf Wallstreet Online.