| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
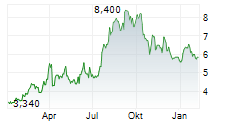
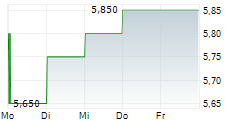
| 5,350 | 5,650 | 17:41 | |
| 5,350 | 5,600 | 17:35 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 03.11.25 | Goldman Sachs initiates Keymed Biosciences stock with Buy rating | 1 | Investing.com | ||
| 03.11.25 | Goldman Sachs: Kaufempfehlung für Keymed Biosciences zum Start der Analyse | - | Investing.com Deutsch | ||
| 25.09.25 | KEYMED BIO-B (02162): 2025 INTERIM REPORT | - | HKEx | ||
| 27.08.25 | Keymed Biosciences (02162.HK) Reports Strong Interim 2025 Results with Accelerated Commercialization and Robust R&D Momentum | 300 | PR Newswire | CHENGDU, China, Aug. 27, 2025 /PRNewswire/ -- Keymed Biosciences Inc. ("Keymed", 02162.HK) announced impressive interim results for 2025, marked by accelerated commercialization and R&D progress.
Financially... ► Artikel lesen | |
| 13.06.25 | Keymed Biosciences Announces the Latest Clinical Trial Results of CM336 Published in the New England Journal of Medicine | 836 | PR Newswire | CHENGDU, China, June 13, 2025 /PRNewswire/ -- On June 12, Keymed Biosciences Inc. (HKEX: 02162) announced that Prof. Jun Shi's research team from the Institute of Hematology and Blood Diseases... ► Artikel lesen | |
| 27.04.25 | Keymed Biosciences gibt die IND-Genehmigung für CM518D1 durch die National Medical Products Administration of China zur Behandlung von Solide Tumoren bekannt | 175 | PR Newswire | CHENGDU, China, 27. April 2025 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) ("Keymed" oder das "Unternehmen") gab vor kurzem bekannt, dass CM518D1, ein... ► Artikel lesen | |
| 25.04.25 | Keymed Biosciences Announces Approval of IND for CM518D1 by the National Medical Products Administration of China for the Treatment of Solid Tumors | 442 | PR Newswire | CHENGDU, China, April 25, 2025 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) ("Keymed" or the "Company") today announced CM518D1, a CDH17-targeted antibody-drug conjugate (ADC) developed... ► Artikel lesen | |
| 07.04.25 | Keymed Biosciences: Daten aus Phase-III-Studie zu Stapokibart gegen saisonale allergische Rhinitis in Nature Medicine veröffentlicht | 356 | PR Newswire | CHENGDU, China, 7. April 2025 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) ("Keymed" oder "Unternehmen") gab heute bekannt, dass die renommierte medizinische... ► Artikel lesen | |
| KEYMED BIOSCIENCES Aktie jetzt für 0€ handeln | |||||
| 05.04.25 | Keymed Biosciences: The Phase III Study Data of Stapokibart for Seasonal Allergic Rhinitis Published in Nature Medicine | 812 | PR Newswire | CHENGDU, China, April 5, 2025 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) ("Keymed" or the "Company") today announced that the prestigious medical journal Nature Medicine has published... ► Artikel lesen | |
| 26.03.25 | Keymed Biosciences Announces Annual Results of 2024 | 241 | PR Newswire | CHENGDU, China, March 26, 2025 /PRNewswire/ -- March. 25, 2025, Keymed Biosciences Inc. (HKEX: 02162) announced its annual results of 2024, along with a corporate... ► Artikel lesen |
10 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 41,320 | -0,17 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| BIONTECH | 93,55 | +0,27 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,692 | -2,83 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| KYMERA THERAPEUTICS | 89,62 | -1,90 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| VIR BIOTECHNOLOGY | 9,660 | +6,15 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| ARCELLX | 113,83 | +0,03 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| BEAM THERAPEUTICS | 28,080 | -1,40 % | Beam Therapeutics Announces $500 Million Strategic Financing Facility with Sixth Street | $100 Million Funded at Close with up to an Additional $400 Million Available Under Facility with Seven-Year Term Financing Bolsters Balance Sheet with Long-term, Non-dilutive Capital to Support Anticipated... ► Artikel lesen | |
| PRAXIS PRECISION MEDICINES | 334,68 | -0,61 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| MODERNA | 45,640 | +0,65 % | Moderna freut sich über eine Zulassungsempfehlung der EMA für einen Kombi-Impfstoff gegen Corona und Grippe | Bei Corona-Impfstoffen war der US-Konzern Moderna noch ein Nachzügler. Anders scheint es nun bei Kombi-Impfstoffen zu laufen, die gleichzeitig vor Corona und der Grippe schützen. Dafür gibt es bisher... ► Artikel lesen | |
| BB BIOTECH | 52,00 | +1,17 % | BB Biotech: Von Innovation zu Wertrealisierung - Der Biotech-Markt in einer neuen Phase | Nach mehreren Jahren, die von erhöhten Kapitalkosten, Bewertungsdruck und ausgeprägter Risikoaversion geprägt waren, hat sich das Umfeld seit dem vergangenen Jahr spürbar stabilisiert. Finanzierungsbedingungen... ► Artikel lesen | |
| IMMUNITYBIO | 8,794 | +6,08 % | ImmunityBio, Inc.: ImmunityBio Reports 700% Year-Over-Year Revenue Growth, Expanded ANKTIVA Approvals in Lung Cancer and Global Commercial Partnerships in 33 Countries with Label Expansion Plans Globally | 2025 Sales Momentum: ANKTIVA net product revenue increased 20% quarter-over-quarter, with full-year net product revenue of $113 million, representing an approximately 700% increase year-over-year... ► Artikel lesen | |
| APOGEE THERAPEUTICS | 70,01 | -0,03 % | Apogee Therapeutics reports FY results | ||
| STRUCTURE THERAPEUTICS | 63,07 | +0,14 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| STRYKER | 331,30 | +1,01 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| OCUGEN | 1,593 | +3,74 % | Ocugen Announces Phase 3 liMeliGhT Enrollment Completion for OCU400, a Novel Modifier Gene Therapy for Broad Retinitis Pigmentosa | Enrollment for liMeliGhT, the first and largest gene therapy registrational trial for broad retinitis pigmentosa (RP) patients, was completed, reflecting strong interest from investigators and patientsTopline... ► Artikel lesen |
KEYMED BIOSCIENCES INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 5,200 Euro und damit -4,59 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu KEYMED BIOSCIENCES INC finden Sie auf Wallstreet Online.