| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,670 | 0,700 | 13:57 | |
| 0,670 | 0,700 | 13:47 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | MaxCyte, Inc: MaxCyte unveils ExPERT DTx, a 96-well electroporation platform to accelerate discovery | 2 | GlobeNewswire (USA) | ||
| 23.02. | MaxCyte, Inc: MaxCyte to Report Fourth Quarter and Full Year 2025 Financial Results on March 24, 2026 | 1 | GlobeNewswire (USA) | ||
| MAXCYTE Aktie jetzt für 0€ handeln | |||||
| 12.01. | MAXCYTE, INC. - 8-K, Current Report | 1 | SEC Filings | ||
| 12.01. | MaxCyte sees Q4 core revenue between $6.6M and $6.7M | 7 | Seeking Alpha | ||
| 12.01. | MaxCyte, Inc: MaxCyte Announces Preliminary Unaudited Fourth Quarter and Full Year 2025 Financial Results | 132 | GlobeNewswire (Europe) | ROCKVILLE, Md., Jan. 12, 2026 (GLOBE NEWSWIRE) -- MaxCyte, Inc., (Nasdaq: MXCT; LSE: MXCT), a leading, cell-engineering focused company providing enabling platform technologies to advance the discovery... ► Artikel lesen | |
| 19.11.25 | MaxCyte stellt auf Stephens-Konferenz Neuausrichtung | 1 | Investing.com Deutsch | ||
| 13.11.25 | MAXCYTE, INC. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 13.11.25 | MaxCyte reports Q3 results | 1 | Seeking Alpha | ||
| 13.11.25 | MaxCyte outlines $17M-$19M annualized savings through restructuring while reiterating SPL revenue guidance | 2 | Seeking Alpha | ||
| 12.11.25 | MaxCyte CFO to step down in first half of 2026 | 2 | Seeking Alpha | ||
| 12.11.25 | MaxCyte CFO Douglas Swirsky to step down in first half of 2026 | 4 | Investing.com | ||
| 12.11.25 | MaxCyte, Inc: MaxCyte Announces Planned CFO Transition in 2026 | 147 | GlobeNewswire (Europe) | ROCKVILLE, Md., Nov. 12, 2025 (GLOBE NEWSWIRE) -- MaxCyte, Inc., (Nasdaq: MXCT), a leading, cell-engineering focused company providing enabling platform technologies to advance the discovery, development... ► Artikel lesen | |
| 11.11.25 | Insights into MaxCyte's Upcoming Earnings | 1 | Benzinga.com | ||
| 05.11.25 | MAXCYTE, INC. - 8-K, Current Report | - | SEC Filings | ||
| 05.11.25 | MaxCyte reports Q3 results | 1 | Seeking Alpha | ||
| 05.11.25 | MaxCyte, Inc: MaxCyte Reports Preliminary Third Quarter 2025 Financial Results and Reiterates Full Year 2025 Revenue Guidance | 194 | GlobeNewswire (Europe) | MaxCyte will now host its earnings conference call on November 12, 2025 Management will present at the Stifel Healthcare Conference on November 11, 2025 and Stephens Investment Conference on November... ► Artikel lesen | |
| 08.10.25 | MaxCyte, Inc: MaxCyte to Report Third Quarter 2025 Financial Results on November 5, 2025 | 3 | GlobeNewswire (USA) | ||
| 06.10.25 | MaxCyte, Inc: MaxCyte Announces Strategic Platform License Agreement with Moonlight Bio to Advance T Cell Therapies for Solid Tumors | 343 | GlobeNewswire (Europe) | ROCKVILLE, Md., Oct. 06, 2025 (GLOBE NEWSWIRE) -- MaxCyte, Inc. (Nasdaq: MXCT), a leading cell-engineering company providing enabling platform technologies to advance the discovery, development, and... ► Artikel lesen | |
| 22.09.25 | MaxCyte announces 34% workforce reduction to cut costs | 1 | Seeking Alpha | ||
| 22.09.25 | MAXCYTE, INC. - 8-K, Current Report | 1 | SEC Filings |
1 2 Weiter >>
28 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,15 | -0,16 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,720 | -2,36 % | Evotec: Das Biotech-Drama geht in die nächste Runde | Die Aktie des Hamburger Wirkstoffforschers Evotec versucht erneut den Befreiungsschlag, doch das Misstrauen am Markt ist greifbar. Nach einer beispiellosen Serie von Rückschlägen - von Management-Skandalen... ► Artikel lesen | |
| MEDIGENE | 0,036 | -10,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,310 | -0,19 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| BIOFRONTERA | 2,780 | +3,73 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| NANOREPRO | 1,540 | -1,60 % | NanoRepro zündet die nächste Story: Mit PHLAS startet der Sprung ins Premium-SkinTech-Geschäft | NanoRepro geht den nächsten strategischen Schritt - und der hat mit klassischen Selbsttests nur noch am Rand zu tun: Mit dem offiziellen Marktstart des Kaltplasmageräts "PHLAS" und dem Livegang des... ► Artikel lesen | |
| CO.DON | 0,016 | -16,22 % | EQS-AFR: CO.DON AG i.l.: Vorabbekanntmachung über die Veröffentlichung von Finanzberichten gemäß §§ 114, 115, 117 WpHG | EQS Vorabbekanntmachung Finanzberichte: CO.DON AG i.l.
/ Vorabbekanntmachung über die Veröffentlichung von Rechnungslegungsberichten
CO.DON AG i.l.: Vorabbekanntmachung über die... ► Artikel lesen | |
| SANGAMO THERAPEUTICS | 0,386 | +4,77 % | Weekly Buzz: ATYR To Meet With FDA, SGMO Aces Fabry Study, AQST's Anaphylm Gets Rejected | PETAH TIKVA (dpa-AFX) - This week's biotech landscape witnessed key FDA meetings being scheduled, IND clearances, complete response letters, sale of oncology assets and clinical trial data readouts... ► Artikel lesen | |
| VIVORYON THERAPEUTICS | 1,466 | +2,09 % | Vivoryon Therapeutics N.V. to Attend and Present in Upcoming Conferences | Vivoryon Therapeutics N.V. to Attend and Present in Upcoming Conferences
Halle (Saale) / Munich, Germany, January 7, 2026 - Vivoryon Therapeutics N.V. (Euronext Amsterdam: VVY; NL00150002Q7) (Vivoryon)... ► Artikel lesen | |
| ABIVAX | 102,20 | -0,58 % | 2 Reasons Abivax Stock Could 10X by 2036 | ||
| IMMATICS | 8,590 | -1,32 % | Immatics Presents IMA203CD8 PRAME Cell Therapy Data from Ongoing Dose Escalation and Shows Promising Initial Anti-tumor Activity in PRAME-Positive Tumors at ESMO-IO 2025 Congress | IMA203CD8 is a second-generation PRAME cell therapy with enhanced pharmacology in ongoing Phase 1a dose escalationManageable tolerability across all dose levelsEncouraging early clinical anti-tumor... ► Artikel lesen | |
| ALDEYRA | 4,606 | -0,41 % | Aldeyra Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| MEDIFAST | 8,840 | -0,87 % | Medifast: OPTAVIA Premieres Second Docuseries Episode "Health by Design: Spain" Exploring Metabolic Health and Longevity | Thousands of Independent Coaches Gather Nationwide to Discuss Solutions to America's Metabolic Health Crisis Medifast (NYSE: MED), the health and wellness company known for its science-backed... ► Artikel lesen | |
| PHARMING | 1,382 | -1,57 % | Pharming Group N.V.: Pharming Group to report fourth quarter and full year 2025 financial results and provide business update on March 12 | Leiden, the Netherlands, February 26, 2026: Pharming Group N.V. ("Pharming" or "the Company") (Euronext Amsterdam: PHARM/Nasdaq: PHAR) confirms that it will report its preliminary (unaudited) financial... ► Artikel lesen | |
| COHERUS ONCOLOGY | 1,384 | -2,40 % | Coherus Oncology, Inc.: Arvind Sood joins Coherus Oncology as Chief Strategy and Corporate Affairs Officer | - Responsible for Corporate Development, Investor Relations, Government Affairs - REDWOOD CITY, Calif., Nov. 06, 2025 (GLOBE NEWSWIRE) -- Coherus Oncology, Inc. (Nasdaq: CHRS), today announced that... ► Artikel lesen |
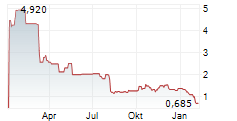
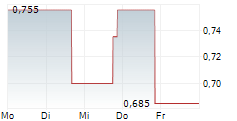
MAXCYTE INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von +0,005 (+0,72 %) liegt der Kurs bei 0,695 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu MAXCYTE INC finden Sie auf Wallstreet Online.