| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 18,200 | 18,600 | 09:45 | |
| 18,200 | 18,600 | 09:12 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | Syndax Pharmaceuticals Inc. (SNDX) on a Strong Footing amid Robust Demand for Key Flagship Drugs | 9 | Insider Monkey | ||
| Fr | Stifel raises Syndax Pharmaceuticals stock price target on drug sales | 3 | Investing.com | ||
| Fr | Syndax Pharmaceuticals: Stifel erhöht Kursziel aufgrund starker Medikamentenumsätze | 6 | Investing.com Deutsch | ||
| Fr | Syndax signals expanding Revuforj market share and targets $10B addressable opportunity while ramping Niktimvo | 7 | Seeking Alpha | ||
| SYNDAX PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| Fr | Syndax Pharma Q4 Loss Decreases | 1 | RTTNews | ||
| Fr | Syndax Q4 2025 slides: revenue surges 50%, profitability path outlined | 2 | Investing.com | ||
| Do | Syndax Pharmaceuticals: EPS verfehlt Schätzungen um 0,19 $ - Umsatz besser als erwartet | 5 | Investing.com Deutsch | ||
| Do | Insights into Syndax Pharmaceuticals Q4 Earnings | 1 | Benzinga.com | ||
| Do | Syndax Pharmaceuticals, Inc.: Syndax Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | 96 | GlobeNewswire (Europe) | - Total revenue of $68.7 million in 4Q25 and $172.4 million in FY2025 - - Revuforj- (revumenib) net revenue of $44.2 million in 4Q25, a 38% increase vs 3Q25, and $124.8 million in FY2025 - - Niktimvo... ► Artikel lesen | |
| Do | Syndax Pharmaceuticals Inc - 10-K, Annual Report | 1 | SEC Filings | ||
| Do | Syndax Pharmaceuticals Inc - 8-K, Current Report | - | SEC Filings | ||
| 12.02. | Syndax auf Guggenheim-Konferenz: Wachstumskurs und strategische Expansion im Fokus | 4 | Investing.com Deutsch | ||
| 04.02. | Syndax Pharmaceuticals, Inc.: Syndax Pharmaceuticals Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4) | 3 | GlobeNewswire (USA) | ||
| 04.02. | Syndax stock maintains momentum as B.Riley reiterates Buy rating | 9 | Investing.com | ||
| 28.01. | XFRA 1T3: WIEDERAUFNAHME/RESUMPTION | 222 | Xetra Newsboard | FOLGENDE(S) INSTRUMENT(E) WIRD/ WERDEN WIEDER IN DEN HANDEL AUFGENOMMEN MIT FOLGENDEM TRADING SCHEDULE.THE FOLLOWING INSTRUMENT(S) IS/ARE RESUMED TRADING WITH FOLLOWING TRADING SCHEDULE:INSTRUMENT NAME... ► Artikel lesen | |
| 12.01. | Syndax Pharmaceuticals Inc - 8-K, Current Report | 6 | SEC Filings | ||
| 07.01. | Syndax Pharmaceuticals, Inc.: Syndax and World Orphan Drug Alliance to Launch a Multi-Regional Managed Access Program, Expanding Access to Revuforj (revumenib) Outside the U.S. | 9 | GlobeNewswire (USA) | ||
| 19.12.25 | Syndax Pharmaceuticals Inc - 8-K, Current Report | 4 | SEC Filings | ||
| 12.12.25 | Syndax Pharmaceuticals, Inc.: Revuforj (revumenib) Named Best New Drug at the Scrip Awards 2025 | 16 | GlobeNewswire (USA) | ||
| 09.12.25 | Syndax stock maintains Buy rating as Revuforj shows real-world success | 5 | Investing.com |
1 2 3 4 Weiter >>
65 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 86,65 | -0,12 % | BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen | |
| EVOTEC | 5,392 | +1,66 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BB BIOTECH | 50,30 | +0,20 % | BB Biotech: Von Innovation zu Wertrealisierung - Der Biotech-Markt in einer neuen Phase | Nach mehreren Jahren, die von erhöhten Kapitalkosten, Bewertungsdruck und ausgeprägter Risikoaversion geprägt waren, hat sich das Umfeld seit dem vergangenen Jahr spürbar stabilisiert. Finanzierungsbedingungen... ► Artikel lesen | |
| MEDIGENE | 0,035 | -10,20 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,095 | +0,97 % | Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 44,800 | +4,44 % | Moderna: Kombi-Impfung für Grippe und Covid erhält Zulassung | Gute Nachrichten für Moderna: Die EU-Arzneimittelbehörde EMA hat grünes Licht gegeben für den ersten Kombi-Impfstoff gegen Corona und Grippe. Der Wirkstoff solle für Menschen ab 50 Jahren zugelassen... ► Artikel lesen | |
| PAION | 0,085 | -8,21 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,380 | -1,13 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 322,50 | -0,65 % | Amgen EVP Sold $20.77M In Company Stock | ||
| EPIGENOMICS | 1,000 | +4,17 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,213 | +0,49 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 330,80 | -0,09 % | Stryker highlights new additions to Mako, Triathlon portfolios at AAOS | ||
| BIOGEN | 154,10 | -2,25 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,770 | +2,97 % | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,830 | -0,35 % | HEIDELBERG PHARMA AG unter Beobachtung |
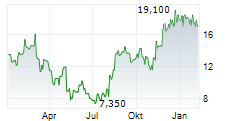
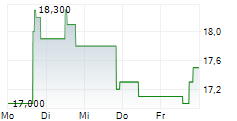
Das Unternehmen SYNDAX PHARMACEUTICALS INC kann der Branche Biotechnologie zugeordnet werden. Mit einer aktuellen Kursveränderung von +0,200 (+1,09 %) liegt der Kurs bei 18,600 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SYNDAX PHARMACEUTICALS INC finden Sie auf Wallstreet Online.