- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| PASITHEA THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 20.02. | Pasithea Therapeutics Corp. - 8-K, Current Report | 4 | SEC Filings | ||
| 30.01. | Pasithea Therapeutics Corp. - S-8, Securities to be offered to employees in employee benefit plans | 1 | SEC Filings | ||
| 13.01. | Pasithea Therapeutics Provides Outlook on PAS-004 Clinical Programs and Data Release Timelines | 135 | GlobeNewswire (Europe) | MIAMI, Jan. 13, 2026 (GLOBE NEWSWIRE) -- Pasithea Therapeutics Corp. (NASDAQ: KTTA) ("Pasithea" or the "Company"), a clinical-stage biotechnology company developing PAS-004, a next-generation oral... ► Artikel lesen | |
| 15.12.25 | Pasithea Therapeutics Corp. - 8-K, Current Report | - | SEC Filings | ||
| 08.12.25 | Pasithea Therapeutics stock initiated with Buy rating at H.C. Wainwright | 2 | Investing.com | ||
| 02.12.25 | Pasithea Therapeutics Announces Closing of $60 Million Public Offering of Common Stock | 3 | GlobeNewswire (USA) | ||
| 28.11.25 | Pasithea Therapeutics Shares Surge Nearly 39% After $60 Mln Equity Raise | 1 | RTTNews | ||
| 28.11.25 | Pasithea Therapeutics Prices $60 Mln Public Offering To Advance MEK Inhibitor, Stock Up | 4 | RTTNews | ||
| 28.11.25 | Pasithea Therapeutics Announces Pricing of $60 Million Public Offering of Common Stock | 2 | GlobeNewswire (USA) | ||
| 26.11.25 | Pasithea Therapeutics Corp. - S-1/A, General form for registration of securities | 14 | SEC Filings | ||
| 25.11.25 | Pasithea Therapeutics Corp. - 8-K, Current Report | - | SEC Filings | ||
| 25.11.25 | ALS Association awards $1 million to study Pasithea's PAS-004 in ALS | 2 | Investing.com | ||
| 24.11.25 | Pasithea reports positive safety data from PAS-004 cancer trial | 7 | Investing.com | ||
| 21.11.25 | Pasithea reports positive PK data for PAS-004 tablet formulation | 1 | Investing.com | ||
| 21.11.25 | Pasithea Therapeutics Announces Positive PAS-004 Tablet Pharmacokinetic (PK) Data in Ongoing Phase 1/1b Trial in Adult NF1 Patients | 260 | GlobeNewswire (Europe) | -- Tablet PK exposure increases proportionally with an increase in dose -- -- More favorable PK properties in tablets enable a lower dose to achieve the same exposure as the capsule formulation, with... ► Artikel lesen | |
| 20.11.25 | Pasithea meldet positive Zwischendaten für MEK-Inhibitor in Krebsstudie | 5 | Investing.com Deutsch | ||
| 20.11.25 | Pasithea reports positive interim data for MEK inhibitor in cancer trial | 1 | Investing.com | ||
| 20.11.25 | Pasithea Therapeutics Announces Positive Phase 1 Data Including Partial Response, Demonstrating Monotherapy Clinical Activity and Favorable Safety Profile for PAS-004 in Advanced Cancer Study | 207 | GlobeNewswire (Europe) | -- Evidence of Monotherapy Activity: Partial response observed in a MEK-rechallenge 3rd-line melanoma patient with BRAF V600E mutation who remains on trial for more than 11 months -- -- A second MEK-rechallenge... ► Artikel lesen | |
| 18.11.25 | Pasithea Therapeutics Corp. - S-1, General form for registration of securities | - | SEC Filings | ||
| 13.11.25 | Pasithea Therapeutics Corp. - 10-Q, Quarterly Report | - | SEC Filings |
1 2 Weiter >>
38 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| OCUGEN | 1,680 | +1,42 % | Ocugen Announces Phase 3 liMeliGhT Enrollment Completion for OCU400, a Novel Modifier Gene Therapy for Broad Retinitis Pigmentosa | Enrollment for liMeliGhT, the first and largest gene therapy registrational trial for broad retinitis pigmentosa (RP) patients, was completed, reflecting strong interest from investigators and patientsTopline... ► Artikel lesen | |
| ABIVAX | 96,50 | -1,53 % | 2 Reasons Abivax Stock Could 10X by 2036 | ||
| ADAPTIVE BIOTECHNOLOGIES | 13,490 | -2,07 % | Why are Analysts Optimistic About Adaptive Biotechnologies (ADPT)? | ||
| GENPREX | 1,980 | -1,49 % | Genprex, Inc.: Genprex Strengthens Intellectual Property Portfolio with Japanese and EU Patents for Reqorsa Gene Therapy in Combination with Immunotherapies to Treat Cancer | Japanese Patent Covers Acclaim-3 Clinical Trial Combining REQORSA Gene Therapy with Genentech, Inc.'sTecentriq®
AUSTIN, Texas, Feb. 23, 2026 /PRNewswire/ -- Genprex... ► Artikel lesen | |
| REZOLUTE | 2,660 | -0,75 % | Rezolute, Inc.: Rezolute Reports Second Quarter Fiscal 2026 Financial Results and Provides Business Update | REDWOOD CITY, Calif., Feb. 12, 2026 (GLOBE NEWSWIRE) -- Rezolute, Inc. (Nasdaq: RZLT) ("Rezolute" or the "Company"), a late-stage rare disease company focused on treating hypoglycemia caused by all... ► Artikel lesen | |
| SPRUCE BIOSCIENCES | 54,00 | -3,57 % | Harbour BioMed Acquires Common Stock in Spruce Biosciences, Deepening Strategic Collaboration | CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SHANGHAI, Jan. 18, 2026 /PRNewswire/ -- Harbour BioMed (HKEX: 02142), a global biopharmaceutical company committed to... ► Artikel lesen | |
| GALECTO | 30,020 | 0,00 % | Galecto, Inc.: Galecto Announces Acquisition of Damora Therapeutics | Acquisition of Damora and concurrent oversubscribed $285 million private investment positions the company to advance potentially best-in-class portfolio to improve outcomes in patients with Myeloproliferative... ► Artikel lesen | |
| UNICYCIVE THERAPEUTICS | 6,990 | +0,58 % | Unicycive Therapeutics, Inc.: Unicycive Therapeutics Announces FDA Acceptance of Oxylanthanum Carbonate (OLC) New Drug Application (NDA) Resubmission | DA assigns Prescription Drug User Fee Act (PDUFA) target date of June 27, 2026Ended 2025 with unaudited cash position of $41.3M with expected runway into 2027 LOS ALTOS, Calif., Jan. 29, 2026 (GLOBE... ► Artikel lesen | |
| FIBROBIOLOGICS | 0,380 | +6,74 % | FibroBiologics, Inc. Announces Issuance of U.S. Patent Covering Fibroblast Cell Therapy for the Treatment of Osteoporosis | HOUSTON, March 02, 2026 (GLOBE NEWSWIRE) -- FibroBiologics, Inc. (Nasdaq: FBLG) ("FibroBiologics" or the "Company"), a clinical-stage biotechnology company with 270+ patents issued and pending with... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,340 | 0,00 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| ARCELLX | 114,05 | +0,22 % | Weekly Buzz: MGNX's LINNET Trial On Hold; Eton Pharmaceuticals, ALUR Get FDA Nod; GILD Snaps Up ACLX | FOSTER CITY (dpa-AFX) - This week, the biotech space witnessed significant milestones, including FDA approvals, oncology drug acquisitions, and collaborations. Positive clinical trial results... ► Artikel lesen | |
| KYMERA THERAPEUTICS | 90,10 | 0,00 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| BEAM THERAPEUTICS | 28,690 | +0,74 % | Beam Therapeutics Reports Fourth Quarter and Year-End 2025 Financial Results and Announces New Liver-Targeted Genetic Disease Program in Phenylketonuria (PKU) | New Program Designed as Platform-based Approach for Direct Correction of Mutations Causing PKU; Investigational New Drug (IND) Filing for BEAM-304 Anticipated in 2026 Updated Phase 1/2 Data and Next... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,420 | -0,98 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| ARCUTIS BIOTHERAPEUTICS | 24,760 | -8,25 % | Arcutis Biotherapeutics, Inc.: Arcutis Announces Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | Q4 2025 net product revenue for ZORYVE (roflumilast) was $127.5 million, an 84% increase compared to Q4 2024, and a 29% increase compared to Q3 2025Full year 2025 net product revenue for ZORYVE was... ► Artikel lesen |
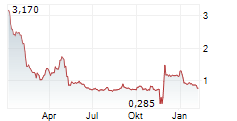
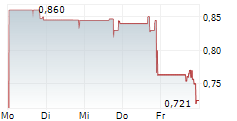
Das Unternehmen PASITHEA THERAPEUTICS CORP kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 0,824 Euro und damit -6,41 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu PASITHEA THERAPEUTICS CORP finden Sie auf Wallstreet Online.