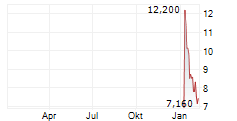- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 20.01. | TRANSTHERA-B (02617): NEXT DAY DISCLOSURE RETURN | 1 | HKEx | ||
| 20.01. | TRANSTHERA-B (02617): COMPLETION OF PLACING OF NEW H SHARES UNDER THE GENERAL MANDATE | 2 | HKEx | ||
| 13.01. | TRANSTHERA-B (02617): PLACING OF NEW H SHARES UNDER GENERAL MANDATE | 5 | HKEx | ||
| 12.01. | TRANSTHERA-B (02617): VOLUNTARY ANNOUNCEMENT PRESENTATION OF CLINICAL DATA ANALYSIS OF TINENGOTINIB MONOTHERAPY IN PATIENTS WITH ADVANCED CHOLANGIOCARCINOMA ... | 1 | HKEx | ||
| 12.01. | XFRA NEW INSTRUMENTS AVAILABLE ON 12.01.2026 | 394 | Xetra Newsboard | The following instruments on XETRA do have their first trading 12.01.2026 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 12.01.2026
Aktien
1 CA2924833023 Enablence Technologies... ► Artikel lesen | |
| 19.12.25 | TransThera Sciences (Nanjing) Inc.: New Drug Application For Tinengotinib Tablets Accepted By The National Medical Products Administration | 154 | PR Newswire | NANJING, China and GAITHERSBURG, Md., Dec. 18, 2025 /PRNewswire/ -- TransThera Sciences Nanjing, Inc. (the "TransThera") announced that the new drug application... ► Artikel lesen | |
| 19.12.25 | TRANSTHERA-B (02617): VOLUNTARY ANNOUNCEMENT NEW DRUG APPLICATION FOR TINENGOTINIB TABLETS ACCEPTED BY THE NATIONAL MEDICAL PRODUCTS ADMINISTRATION | 2 | HKEx | ||
| 17.12.25 | TRANSTHERA-B (02617): VOLUNTARY ANNOUNCEMENT PUBLICATION OF CLINICAL RESULTS OF OUR CORE PRODUCT TINENGOTINIB AGAINST CHOLANGIOCARCINOMA | - | HKEx | ||
| TRANSTHERA SCIENCES Aktie jetzt für 0€ handeln | |||||
| 03.12.25 | TRANSTHERA-B (02617): VOLUNTARY ANNOUNCEMENT INCLUSION OF TINENGOTINIB TABLETS IN THE LIST OF PRODUCTS FOR PRIORITY REVIEW BY THE NATIONAL MEDICAL PRODUCTS ... | - | HKEx | ||
| 03.11.25 | Neurocrine pens $880M deal with China's TransThera for red-hot immunology target | 12 | FierceBiotech | ||
| 03.11.25 | TRANSTHERA-B (02617): VOLUNTARY ANNOUNCEMENT COLLABORATION WITH NEUROCRINE | 4 | HKEx | ||
| 20.10.25 | TRANSTHERA-B (02617): INSIDE INFORMATION ANNOUNCEMENT PROPOSED IMPLEMENTATION OF THE H SHARE FULL CIRCULATION BY THE COMPANY | 1 | HKEx | ||
| 25.09.25 | A wild ride for TransThera investors: what's the story? | 1 | Bamboo Works | ||
| 19.09.25 | TRANSTHERA-B (02617): INTERIM REPORT 2025 | 2 | HKEx | ||
| 16.09.25 | TRANSTHERA-B (02617): VOLUNTARY ANNOUNCEMENT UNUSUAL PRICE AND TRADING VOLUME MOVEMENTS | 1 | HKEx | ||
| 10.09.25 | TRANSTHERA-B (02617): VOLUNTARY ANNOUNCEMENT PHASE II IND APPROVAL OF TINENGOTINIB IN COMBINATION WITH FULVESTRANT FOR THE TREATMENT OF PREVIOUSLY TREATED ... | - | HKEx | ||
| 04.09.25 | TRANSTHERA-B (02617): VOLUNTARY ANNOUNCEMENT FIRST PATIENT DOSED IN PHASE II CLINICAL TRIAL OF TINENGOTINIB IN COMBINATION WITH AKESO'S (CADONILIMAB, ... | - | HKEx |
17 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 41,295 | -0,23 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| BIONTECH | 92,75 | -0,59 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,686 | -2,94 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,600 | +5,49 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| KYMERA THERAPEUTICS | 90,10 | -1,38 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| BEAM THERAPEUTICS | 28,440 | -0,14 % | Beam Therapeutics Announces $500 Million Strategic Financing Facility with Sixth Street | $100 Million Funded at Close with up to an Additional $400 Million Available Under Facility with Seven-Year Term Financing Bolsters Balance Sheet with Long-term, Non-dilutive Capital to Support Anticipated... ► Artikel lesen | |
| ARCELLX | 114,03 | +0,21 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| ADMA BIOLOGICS | 16,680 | +7,06 % | ADMA Biologics Announces $200 Mln Capital Return Plan | ||
| STRUCTURE THERAPEUTICS | 63,38 | +0,64 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| COGENT BIOSCIENCES | 38,670 | -0,51 % | Cogent Biosciences, Inc.: Cogent Biosciences Highlights Additional Data with Six Bezuclastinib Posters from SUMMIT Trial at 2026 AAAAI Annual Meeting | Bezuclastinib mean TSS reduction deepens to -32.0 points at 48 weeks of treatment with further improvement shown across all measured symptoms99% of patients achieve >50% reduction in serum tryptase... ► Artikel lesen | |
| ERASCA | 14,150 | +3,66 % | Erasca, Inc.: Erasca Announces Issuance of a U.S. Patent Covering Pan-KRAS Inhibitor ERAS-4001 | The issued patent provides intellectual property protection for ERAS-4001 and related compositions until at least 2043 Expands Erasca's diversified IP portfolio for RAS-driven cancers Initial Phase... ► Artikel lesen | |
| NUVALENT | 101,93 | +0,01 % | Nuvalent, Inc.: Nuvalent Outlines Recent Pipeline Progress, Reiterates Key Anticipated Milestones, and Reports Fourth Quarter and Full Year 2025 Financial Results | Commercial preparations well underway to support potential U.S. launch of zidesamtinib in TKI pre-treated advanced ROS1-positive NSCLC population, pending FDA review... ► Artikel lesen | |
| IMMUNITYBIO | 9,276 | +11,89 % | ImmunityBio, Inc.: ImmunityBio Reports 700% Year-Over-Year Revenue Growth, Expanded ANKTIVA Approvals in Lung Cancer and Global Commercial Partnerships in 33 Countries with Label Expansion Plans Globally | 2025 Sales Momentum: ANKTIVA net product revenue increased 20% quarter-over-quarter, with full-year net product revenue of $113 million, representing an approximately 700% increase year-over-year... ► Artikel lesen | |
| PRAXIS PRECISION MEDICINES | 337,18 | +0,13 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| MODERNA | 46,530 | +2,61 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen |
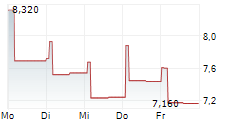
Das Unternehmen TRANSTHERA SCIENCES NANJING INC kann der Branche Biotechnologie zugeordnet werden. Mit einer aktuellen Kursveränderung von -0,550 (-7,86 %) liegt der Kurs bei 6,450 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu TRANSTHERA SCIENCES NANJING INC finden Sie auf Wallstreet Online.