| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 15,200 | 15,600 | 11.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 00:02 | MESOBLAST LIMITED: High Survival Rates with Ryoncil in SR-aGvHD EIND Program | 1 | ASX | ||
| So | MESOBLAST LIMITED: Becoming a substantial holder | 2 | ASX | ||
| 29.01. | Could Mesoblast shares hit $4.45 in 2026? | 15 | The Motley Fool Australia | ||
| 29.01. | Mesoblast posts revenue jump and new funding: Key takeaways for investors | 7 | The Motley Fool Australia | ||
| 29.01. | Mesoblast Limited: Ryoncil Net Revenues Increase for the Quarter to US$30M | 172 | GlobeNewswire (Europe) | NEW YORK, Jan. 28, 2026 (GLOBE NEWSWIRE) -- Mesoblast Limited (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today provided highlights of its recent... ► Artikel lesen | |
| MESOBLAST LIMITED ADR Aktie jetzt für 0€ handeln | |||||
| 29.01. | MESOBLAST LIMITED: Quarterly Activities/Appendix 4C Cash Flow Report | 1 | ASX | ||
| 28.01. | MESOBLAST LTD - 6-K, Report of foreign issuer | 4 | SEC Filings | ||
| 27.01. | Mesoblast Says Most Children Survive After Early Use Of FDA-Approved Cell Therapy For Organ Transplant Related Complication | 2 | Benzinga.com | ||
| 27.01. | Mesoblast Limited: Real-World Commercial Experience with Ryoncil Shows 84% Survival of Children with SR-aGvHD After Completing 28-Days of Treatment | 6 | GlobeNewswire (USA) | ||
| 27.01. | MESOBLAST LIMITED: Real World Experience with Ryoncil Shows 84% Early Survival | 3 | ASX | ||
| 26.01. | MESOBLAST LIMITED: Ceasing to be a substantial holder | - | ASX | ||
| 26.01. | MESOBLAST LIMITED: Becoming a substantial holder | - | ASX | ||
| 20.01. | FDA Signals Support For Mesoblast's Cell Therapy For Chronic Back Pain | 2 | Benzinga.com | ||
| 19.01. | Mesoblast Receives Encouraging FDA Feedback | 6 | Finance News Network | ||
| 19.01. | Mesoblast dips on confusion around FDA advice on stem cells for back pain | 5 | The Market Herald Australia | ||
| 19.01. | Mesoblast just cleared a key FDA hurdle. So why are investors exiting? | 7 | The Motley Fool Australia | ||
| 19.01. | Mesoblast Gets FDA Feedback On Potential BLA Filing For Rexlemestrocel-L In Chronic Low Back Pain | 5 | RTTNews | ||
| 19.01. | Mesoblast Limited: FDA Acknowledges Effects on Pain Intensity Favor Rexlemestrocel-L, Confirms 12-Month Reduction in Back Pain Supports Product Efficacy | 698 | GlobeNewswire (Europe) | NEW YORK, Jan. 18, 2026 (GLOBE NEWSWIRE) -- Mesoblast Limited (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today provided feedback received from... ► Artikel lesen | |
| 19.01. | MESOBLAST LIMITED: FDA Confirms Path to Approval for CLBP | 1 | ASX | ||
| 16.01. | MESOBLAST LTD - 6-K, Report of foreign issuer | 3 | SEC Filings |
1 2 3 4 5 Weiter >>
164 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 92,00 | -0,22 % | BioNTech-Aktie: Chancen im Schlüsseljahr 2026 | BioNTech verstärkt seit Anfang 2026 seine strategische Neuausrichtung weg vom Corona-Impfstoff-Geschäft hin zu Onkologie und Immuntherapien. Das Jahr 2026 ist explizit als "Schlüsseljahr" für klinische... ► Artikel lesen | |
| EVOTEC | 5,992 | -2,92 % | Evotec-Aktie: War's das schon wieder oder geht's weiter hoch? | Nach dem kräftigen Kurssprung am Dienstag aufgrund einer Analysten-Empfehlung legt die Evotec-Aktie wieder leicht den Rückwärtsgang ein und notiert aktuell bei 6,25 €. War's das schon wieder? Oder bleibt... ► Artikel lesen | |
| BB BIOTECH | 49,700 | -0,40 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,040 | -6,51 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,000 | -0,43 % | JEFFERIES stuft QIAGEN NV auf 'Buy' | NEW YORK (dpa-AFX Analyser) - Das Analysehaus Jefferies hat die Einstufung für Qiagen mit einem Kursziel von 57 US-Dollar auf "Buy" belassen. Umsatz und Ergebnis des vierten Quartals hätten die Erwartungen... ► Artikel lesen | |
| MODERNA | 34,060 | -0,16 % | Rückschlag für MODERNA | Die US-Arzneimittelbehörde FDA hat die Prüfung eines Zulassungsantrags von MODERNA für dessen experimentellen mRNA-Grippeimpfstoff mRNA-1010 abgelehnt. Statt in das inhaltliche Prüfverfahren einzusteigen... ► Artikel lesen | |
| PAION | 0,160 | -11,11 % | PAION AG zeigt strategische Stärke | ||
| VALNEVA | 4,108 | -1,86 % | VALNEVA Declaration of shares and voting rights: January 31, 2026 | ||
| AMGEN | 308,05 | -0,21 % | Märkte am Morgen: Gold, Silber, Bitcoin, Amgen, Alphabet, AMD, SAP, Siemens Healthineers, BASF | Das Umfeld an den Aktienmärkten ist aktuell alles andere als einfach. Das hat sich gestern mal wieder deutlich gezeigt. Der DAX geriet nach guten Start unter Druck und schloss 0,72 Prozent tiefer bei... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 7,762 | +2,78 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 306,70 | +0,10 % | Stryker launches T2 Alpha humerus nailing system, expands trauma nailing portfolio | ||
| BIOGEN | 161,10 | -0,43 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,400 | +0,84 % | Biofrontera's Ameluz SNDA For Superficial Basal Cell Carcinoma Accepted By FDA | ||
| HEIDELBERG PHARMA | 3,100 | +0,32 % | Heidelberg Pharma erhält Meilensteinzahlung von Takeda | Die Heidelberg Pharma AG hat im Rahmen einer bestehenden Lizenzvereinbarung eine Meilensteinzahlung von Takeda erhalten. Die Zahlung wurde mit der Dosierung des ersten Patienten in einer klinischen... ► Artikel lesen |
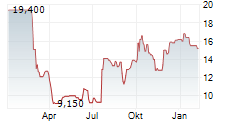
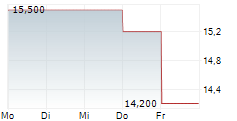
Das Unternehmen MESOBLAST LIMITED ADR kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 14,300 Euro und damit +0,70 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu MESOBLAST LIMITED ADR finden Sie auf Wallstreet Online.