Chart-Typ:
Zeitraum:
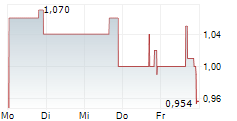
| T1 Energy Inc.: Update on T1 Energy FEOC Compliance Efforts | AUSTIN, Texas and NEW YORK, Dec. 30, 2025 (GLOBE NEWSWIRE) -- T1 Energy Inc. (NYSE: TE) ("T1," "T1 Energy," or the "Company") announced that today it has concluded a series of transactions with Trina... ► Artikel lesen |
| T1 Energy Inc.: T1 Executes First Sale of Section 45X Tax Credits | AUSTIN, Texas and NEW YORK, Dec. 30, 2025 (GLOBE NEWSWIRE) -- T1 Energy Inc. (NYSE: TE) ("T1," "T1 Energy," or the "Company") announced this morning that the Company has completed a $160 million sale... ► Artikel lesen |
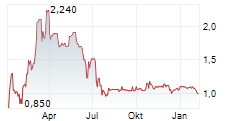
| Trina Solar completes sale of 5 GW U.S. module plant to T1 Energy | Trina Solar has completed the sale of its 5 GW US solar module factory to T1 Energy, exiting direct manufacturing while retaining a minority equity stake.Trina Solar has completed the sale of its 5... ► Artikel lesen |
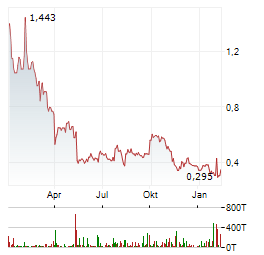
| Niu Technologies Provides Fourth Quarter and Full Year 2025 Sales Volume Update | BEIJING, Jan. 05, 2026 (GLOBE NEWSWIRE) -- Niu Technologies ("NIU" or the "Company") (NASDAQ: NIU), the world's leading provider of smart urban mobility solutions, today provided its sales volume... ► Artikel lesen |
| Niu Technologies Announces Unaudited Third Quarter 2025 Financial Results | -- Third Quarter Revenues of RMB 1,693.9 million, increase 65.4% year over year -- Third Quarter Net Income of RMB 81.7 million, compared to net loss of RMB 40.9 million in the same period of last... ► Artikel lesen |
| NIU TECHNOLOGIES: Beim Absatz ein matt-glänzendes Q3. | Der 2014 gegründete chinesische Hersteller elektrisch betriebener Zweiräder (Sortiment von Motorrädern über Scooter bis Bikes) meldete für das dritte Quartal ein Absatzplus von 49 % zum Vorjahr nach... ► Artikel lesen |
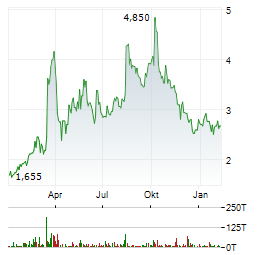
| Weekly Buzz: ATYR To Meet With FDA, SGMO Aces Fabry Study, AQST's Anaphylm Gets Rejected | PETAH TIKVA (dpa-AFX) - This week's biotech landscape witnessed key FDA meetings being scheduled, IND clearances, complete response letters, sale of oncology assets and clinical trial data readouts... ► Artikel lesen |
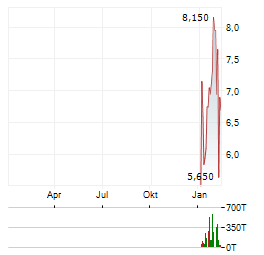
| Sangamo Therapeutics, Inc.: Sangamo Therapeutics Initiates Rolling Submission of BLA to U.S. FDA for ST-920 in Fabry Disease | STAAR study demonstrated positive mean annualized estimated glomerular filtration rate (eGFR) slope at 52-weeks across all dosed patients in the study, which U.S. Food and Drug Administration (FDA)... ► Artikel lesen |
| Sangamo Therapeutics, Inc.: Sangamo Therapeutics Receives U.S. FDA Fast Track Designation for ST-503 for the Treatment of Small Fiber Neuropathy | RICHMOND, Calif., Dec. 02, 2025 (GLOBE NEWSWIRE) -- Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a genomic medicine company, today announced that the U.S. Food and Drug Administration (FDA) has granted... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GIFTIFY INC finden Sie auf Wallstreet Online.