WESTON (dpa-AFX) - Eisai Co., Ltd. (ESALY.PK, ESALF.PK, 4523.T) and Biogen Inc. (BIIB) announced the U.S. availability of LEQEMBI IQLIK (lecanemab-irmb) as a subcutaneous injection for maintenance dosing in the treatment of Alzheimer's disease. This option is intended for patients in the early stages of Alzheimer's disease, including those with Mild Cognitive Impairment (MCI) or mild dementia.
Following 18 months of intravenous (IV) LEQEMBI treatment at 10 mg/kg every two weeks, patients now have two maintenance options: continue IV infusions at 10 mg/kg once every four weeks; transition to a weekly 360 mg subcutaneous injection using the LEQEMBI IQLIK autoinjector.
The companies have also launched the LEQEMBI Companion program, an initiative rooted in both companies' commitment to providing access to LEQEMBI and resources for people living with early Alzheimer's disease. The program aims to provide expanded resources that support patients throughout their LEQEMBI treatment journey, from initiation through maintenance therapy.
Eisai serves as the lead for lecanemab's development and regulatory submissions globally with Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority.
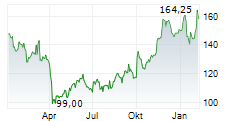
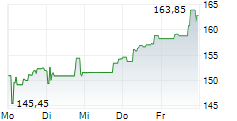
BIIB closed Monday's regular trading at $154.05 down $5.83 or 3.65%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News