WESTON (dpa-AFX) - Eisai Co., Ltd. (ESALY, ESALF, 4523.T) and Biogen Inc. (BIIB) announced that the U.S. Food and Drug Administration has accepted for review Eisai's Supplemental Biologics License Application (sBLA) for lecanemab-irmb (U.S. brand name: LEQEMBI) subcutaneous autoinjector (SC-AI), LEQEMBI IQLIK, as a weekly starting dose.
LEQEMBI is indicated for the treatment of Alzheimer's disease (AD) in patients with Mild Cognitive Impairment (MCI) or mild dementia stage of disease (collectively referred to as early AD).
The sBLA has been granted Priority Review, with a Prescription Drug User Fee Act (PDUFA) action date of May 24, 2026.
If approved, LEQEMBI IQLIK would be the first and only anti-amyloid treatment to offer at-home injection options for initiation and maintenance dosing for this progressive, relentless disease.
LEQEMBI is currently approved in 53 countries and regions and is under regulatory review in 7 countries. In August 2025, the US FDA approved LEQEMBI IQLIK 360 mg for weekly subcutaneous maintenance dosing after 18 months of IV treatment every two weeks.
Eisai serves as the lead for lecanemab's development and regulatory submissions globally, with Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority.
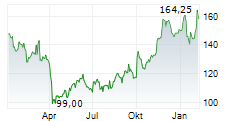
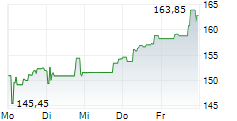
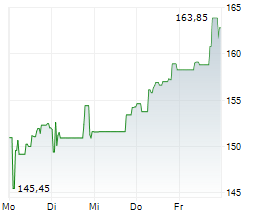
BIIB closed at $171.59 on January 23, 2026, down $2.21 or 1.27% at the end of regular trading. In overnight trading on January 25, 2026 (8:00 PM EST), the stock slipped further to $169.76, a decline of $1.83 or 1.07%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News