- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 14:13 | CSPC PHARMA (01093): VOLUNTARY ANNOUNCEMENT - PACLITAXEL PROTEIN-BOUND PARTICLES FOR INJECTABLE SUSPENSION, RAPID SUSPENSION (ALBUMIN-BOUND) (SYHX2011G1) ... | 1 | HKEx | ||
| 14:09 | CSPC PHARMA (01093): LIST OF DIRECTORS AND THEIR ROLE AND FUNCTION | 2 | HKEx | ||
| CSPC PHARMACEUTICAL GROUP LTD ADR Aktie jetzt für 0€ handeln | |||||
| 14:08 | CSPC PHARMA (01093): APPOINTMENT OF AN EXECUTIVE DIRECTOR | 2 | HKEx | ||
| Do | CSPC PHARMA (01093): CONNECTED TRANSACTION - DISPOSAL OF 30.0704% EQUITY INTEREST IN THE TARGET COMPANY | 6 | HKEx | ||
| 16.02. | CSPC PHARMA (01093): VOLUNTARY ANNOUNCEMENT - GLP-1/GIP RECEPTOR DUAL-BIASED AGONIST POLYPEPTIDE LONG-ACTING INJECTION (SYH2082 INJECTION) OBTAINS CLINICAL ... | 5 | HKEx | ||
| 16.02. | CSPC PHARMA (01093): VOLUNTARY ANNOUNCEMENT - ROPIVACAINE LONG-ACTING INJECTION (SYH9089 INJECTION) OBTAINS CLINICAL TRIAL APPROVAL IN CHINA | 2 | HKEx | ||
| 16.02. | Nomura Lifts CSPC PHARMA TP to $12.04, Keeps Buy Rating | 5 | AASTOCKS | ||
| 10.02. | HSI Closes at 27,183, Up 155 pts; HSTI Closes at 5,451, Up 33 pts; CSPC PHARMA Up over 5%; ZTO EXPRESS-W, WH GROUP, BOC HONG KONG, WHARF REIC, HANG LUNG PPT Hit New Highs; Market Turnover Rises | 8 | AASTOCKS | ||
| 03.02. | HSI Closes at 26,834, Up 59 pts; HSTI Closes at 5,467, Down 59 pts; CSPC PHARMA Up over 8%; NEW ORIENTAL, HSBC HOLDINGS, MTR CORPORATION, POWER ASSETS, CIMC Hit New Highs; Market Turnover Rises | 2 | AASTOCKS | ||
| 02.02. | CSPC PHARMA (01093): VOLUNTARY ANNOUNCEMENT - CLEVIDIPINE INJECTABLE EMULSION OBTAINS DRUG REGISTRATION APPROVAL | 2 | HKEx | ||
| 02.02. | CICC Raises CSPC PHARMA (01093.HK) TP to $12, Anticipates Continued Out-licensing Realization | 13 | AASTOCKS | ||
| 02.02. | BofAS Adds CSPC PHARMA (01093.HK) TP to $9.1, Keeps Underperform Rating on Sales Pressure | 7 | AASTOCKS | ||
| 30.01. | AstraZeneca returns to China's CSPC Pharma for $4.7B obesity deal | 5 | FierceBiotech | ||
| 30.01. | AstraZeneca deepens Chinese ties, signs $18.5bn CSPC Pharma obesity deal | 4 | pharmaphorum | ||
| 30.01. | AstraZeneca strikes up to $18.5B obesity drug deal with China's CSPC Pharmaceutical | 4 | Seeking Alpha | ||
| 30.01. | AstraZeneca Strikes Deal With CSPC Pharmaceutical To Boost Obesity And Diabetes Therapies | 661 | AFX News | LONDON (dpa-AFX) - AstraZeneca PLC (AZN) said on Friday it has entered a strategic collaboration with CSPC Pharmaceuticals, paying $1.2 billion upfront to expand its weight-management pipeline.... ► Artikel lesen | |
| 30.01. | AstraZeneca PLC: AstraZeneca agrees obesity and T2D deal with CSPC Pharmaceutical | 373 | GlobeNewswire (Europe) | 30 January 2026
AstraZeneca enhances its weight management portfolio through collaboration agreement with CSPC Pharmaceuticals
Agreement includes eight programmes, including a clinical-ready asset... ► Artikel lesen | |
| 30.01. | AstraZeneca zahlt CSPC Pharma bis zu 18,5 Milliarden Dollar für Adipositas-Medikamente | 14 | Investing.com Deutsch | ||
| 30.01. | China's CSPC Pharmaceutical, AstraZeneca partner on long-acting obesity drugs | 5 | Seeking Alpha | ||
| 30.01. | CSPC PHARMA (01093): VOLUNTARY ANNOUNCEMENT - ENTERING INTO A STRATEGIC COLLABORATION AND LICENSE AGREEMENT WITH ASTRAZENECA FOR THE DEVELOPMENT OF INNOVATIVE ... | 2 | HKEx |
1 2 3 4 5 Weiter >>
87 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,890 | -2,28 % | Märkte am Morgen: Bayer, SAP, Scout24, Knorr-Bremse, thyssenkrupp, Bitcoin | Der DAX hat eine turbulente Woche hinter sich. Am Freitag gab es noch einmal einen ordentlichen Schub nach oben. In den USA hatte der Supreme Court die Zölle von Donald Trump für unrechtmäßig erklärt.... ► Artikel lesen | |
| SANOFI | 82,03 | +0,35 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 25,030 | +0,89 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ELI LILLY | 896,80 | +0,66 % | Opening Bell: Coca-Cola, Nvidia, Eli Lilly, Novo Nordisk, Vistra Energy, Energy Fuels | An der Wall Street richtet sich die Aufmerksamkeit mal wieder auf die Zollpolitik von Donald Trump. Der Supreme Court hatte am Freitag die bisherigen Maßnahmen gekippt. Trump mit neuen Zöllen unter... ► Artikel lesen | |
| MERCK & CO | 104,80 | 0,00 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen | |
| ASTRAZENECA | 174,90 | -0,88 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TEVA | 29,000 | +1,05 % | Teva Pharmaceutical Industries Ltd: Teva to Present at the Upcoming Investor Conferences in March | PARSIPPANY, N.J. and TEL AVIV, Israel, Feb. 24, 2026 (GLOBE NEWSWIRE) -- Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) today announced that Richard Francis, Teva's President and CEO, will... ► Artikel lesen | |
| VERTEX PHARMACEUTICALS | 422,25 | +0,43 % | Vertex Pharmaceuticals Inc. Reports Rise In Q4 Bottom Line | CAMBRIDGE (MASSACHUSETTS) (dpa-AFX) - Vertex Pharmaceuticals Inc. (VRTX) reported earnings for its fourth quarter that Increases, from last yearThe company's earnings came in at $1.191 billion... ► Artikel lesen | |
| DERMAPHARM | 39,900 | +1,53 % | Dermapharm: Spannend für langfristig orientierte Investoren | Am 17. März wird Dermapharm Zahlen für 2025 präsentieren. Erwartet wird von Dermapharm ein Umsatz von 1,16 Milliarden Euro bis 1,2 Milliarden Euro. Das bereinigte EBITDA sieht die Gesellschaft bei 322... ► Artikel lesen | |
| VIATRIS | 12,720 | +0,83 % | Viatris Posts Narrower Loss In Q4; Expects Workforce Reduction Of Up To Approx. 10% | WASHINGTON (dpa-AFX) - Viatris Inc. (VTRS) reported a fourth quarter net loss of $340.1 million or $0.30 per share compared to a loss of $516.5 million or $0.43 per share, prior year. The company... ► Artikel lesen | |
| INCYTE | 85,76 | +0,07 % | Lilly and Incyte's Olumiant Recommended in EU for Adolescents with Severe Alopecia Areata | ||
| EDWARDS LIFESCIENCES | 73,20 | 0,00 % | Edwards Lifesciences Corp - 10-K, Annual Report | ||
| NOVO NORDISK | 31,725 | +0,16 % | Novo Nordisk: Analyst senkt Kursziel um 35 Prozent - dieser Support muss jetzt halten | Kräftig im Minus ist die Aktie von Novo Nordisk heute in den Handel gestartet. Neben der allgemeinen Marktschwäche sorgte insbesondere eine Abstufung aus den USA für ordentlich Druck. Positiv wurde... ► Artikel lesen | |
| MERCK KGAA | 126,40 | +0,60 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| PFIZER | 23,430 | +0,17 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen |
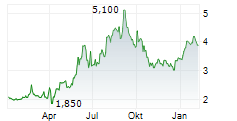
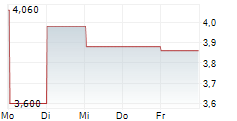
CSPC PHARMACEUTICAL GROUP LTD ADR gehört der Branche Pharma an. Die relative Kursveränderung beträgt aktuell -7,11 % bei einem Aktienkurs von 3,660 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CSPC PHARMACEUTICAL GROUP LTD ADR finden Sie auf Wallstreet Online.