- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | Needham lowers Day One Biopharmaceuticals price target on costs | 3 | Investing.com | ||
| Mi | H.C. Wainwright lowers Day One Biopharmaceuticals price target to $22 | 1 | Investing.com | ||
| Mi | H.C. Wainwright senkt Kursziel für Day One Biopharmaceuticals auf 22 US-Dollar | 2 | Investing.com Deutsch | ||
| Mi | Day One BioPharmaceuticals reiterates $225M-$250M OJEMDA revenue target as pipeline expands with Emi-Le integration | 1 | Seeking Alpha | ||
| Di | Day One BioPharmaceuticals reports Q4 results | 6 | Seeking Alpha | ||
| DAY ONE BIOPHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| Di | Day One Biopharmaceuticals, Inc. - 10-K, Annual Report | 2 | SEC Filings | ||
| Di | Day One Biopharmaceuticals, Inc.: Day One Reports Fourth Quarter and Full Year 2025 Financial Results and Reaffirms 2026 Outlook and Revenue Guidance | 79 | GlobeNewswire (Europe) | OJEMDA 2025 momentum reflected by Q4 and full year net product revenues of $52.8 million and $155.4 million, respectively 2026 U.S. net product revenue projected at $225 - $250 million Expanded pipeline... ► Artikel lesen | |
| Di | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| Di | Nach Mersana-Deal: Day One Biopharmaceuticals legt Quartalszahlen vor - Wachstum im Fokus | 2 | Investing.com Deutsch | ||
| Mo | Day One BioPharmaceuticals Q4 2025 Earnings Preview | 4 | Seeking Alpha | ||
| 13.01. | Day One Biopharmaceuticals stock initiated with Buy rating at TD Cowen | 11 | Investing.com | ||
| 12.01. | Day One Biopharmaceuticals: Starke Umsatzzahlen für 2025 und positive Prognose beflügeln Aktie | 6 | Investing.com Deutsch | ||
| 12.01. | Planet Labs PBC, Day One Biopharmaceuticals, Rxsight, SunOpta And Other Big Stocks Moving Higher On Monday | 4 | Benzinga.com | ||
| 11.01. | Day One Biopharmaceuticals, Inc.: Day One Announces Preliminary 2025 OJEMDA Net Product Revenue And Provides 2026 Net Product Revenue Guidance | 222 | GlobeNewswire (Europe) | Preliminary 2025 net product revenue of $155.4 million, representing 172% year-over-year growth; OJEMDA 2026 U.S. net product revenue projected to be $225 - $250 million Company to present on corporate... ► Artikel lesen | |
| 06.01. | Day One Biopharmaceuticals, Inc.: Day One Completes Acquisition of Mersana Therapeutics | 442 | GlobeNewswire (Europe) | BRISBANE, Calif., Jan. 06, 2026 (GLOBE NEWSWIRE) -- Day One Biopharmaceuticals, Inc. (Nasdaq: DAWN) ("Day One"), a biopharmaceutical company dedicated to developing and commercializing targeted therapies... ► Artikel lesen | |
| 06.01. | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 25.11.25 | Day One Biopharmaceuticals stock maintains Buy rating at H.C. Wainwright | 5 | Investing.com | ||
| 25.11.25 | Day One Biopharmaceuticals: H.C. Wainwright bekräftigt "Buy"-Rating mit hohem Kursziel | 19 | Investing.com Deutsch | ||
| 13.11.25 | Day One Biopharma Expands Oncology Pipeline With $285 Million Mersana Buyout | 5 | Benzinga.com | ||
| 13.11.25 | Mersana Therapeutics To Be Acquired By Day One Biopharmaceuticals For Up To $285 Mln | 19 | RTTNews |
1 2 Weiter >>
31 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| CRISPR THERAPEUTICS | 50,50 | -0,98 % | Crispr Therapeutics gains amid takeover speculation | ||
| VIKING THERAPEUTICS | 28,340 | -1,19 % | Viking Therapeutics: The High-Stakes Weight Loss Contender | ||
| MAINZ BIOMED | 0,748 | +2,78 % | Mainz BioMed NV: Mainz Biomed to Present at the Pancreatic Cancer Session at Digestive Disease Week, Demonstrating Effectiveness of mRNA Biomarkers in Blood Samples | ||
| INTELLIA THERAPEUTICS | 11,645 | -0,13 % | Intellia Therapeutics, Inc.: Intellia Therapeutics Announces Fourth Quarter and Full-Year 2025 Financial Results and Business Updates | HAELO Phase 3 clinical data for lonvo-z in HAE expected by mid-2026; BLA submission in second half of 2026; anticipated U.S. launch in first half of 2027Process underway to reactivate global sites... ► Artikel lesen | |
| TEMPUS AI | 45,000 | -0,44 % | Tempus AI Quartalszahlen: 90 % Umsatzplus bestätigen KI-Trend in der Medizin | CHICAGO, Illinois (IT-Times) - Das Diagnose-Technologie-Unternehmen Tempus AI hat seine Ergebnisse für das vierte Quartal 2025 veröffentlicht und ein rasantes Umsatzwachstum verzeichnet. Tempus AI -... ► Artikel lesen | |
| BIOCRYST PHARMACEUTICALS | 7,342 | -0,92 % | BioCryst Pharmaceuticals, Inc.: BioCryst Reports Full Year 2025 Financial Results and Provides Business Update | -Full year 2025 ORLADEYO® net revenue of $601.8 million (+38% y-o-y; +43% y-o-y excluding European ORLADEYO revenue following the sale of the European ORLADEYO business to Neopharmed Gentili S.p.A.... ► Artikel lesen | |
| BIOMARIN PHARMACEUTICAL | 52,88 | +1,23 % | FDA Expands BioMarin's PALYNZIQ Approval To Include Pediatric PKU Patients Ages 12 And Up | WASHINGTON (dpa-AFX) - BioMarin Pharmaceutical Inc. (BMRN) announced Friday that the U.S. Food and Drug Administration has approved the company's supplemental Biologics License Application for... ► Artikel lesen | |
| SAREPTA THERAPEUTICS | 14,155 | -0,18 % | Sarepta Therapeutics Stock: A Deep Dive Into Analyst Perspectives (5 Ratings) | ||
| EXELIXIS | 37,260 | -0,11 % | 3 Reasons Exelixis Stock Could Deliver Market-Beating Returns Over the Next Decade | ||
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,438 | +0,86 % | PACIFIC BIOSCIENCES OF CALIFORNIA, INC. - S-8, Securities to be offered to employees in employee benefit plans | ||
| CARDIOL THERAPEUTICS | 0,920 | +6,11 % | Cardiol Therapeutics Inc.: Cardiol Therapeutics to Present at TD Cowen 46th Annual Health Care Conference | Toronto, Ontario--(Newsfile Corp. - February 25, 2026) - Cardiol Therapeutics Inc. (NASDAQ: CRDL) (TSX: CRDL) ("Cardiol" or the "Company"), a late-stage life sciences company focused on advancing... ► Artikel lesen |
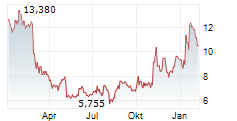
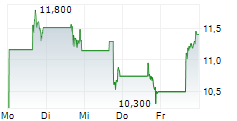
DAY ONE BIOPHARMACEUTICALS INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 10,610 Euro und damit -0,38 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu DAY ONE BIOPHARMACEUTICALS INC finden Sie auf Wallstreet Online.