| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
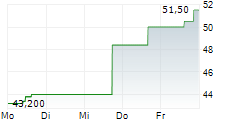
| 50,50 | 51,50 | 07.02. | |
| 50,50 | 51,00 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| GSK PLC ADR Aktie jetzt für 0€ handeln | |||||
| Fr | GSK shares uptick for seven consecutive sessions | 8 | Seeking Alpha | ||
| Fr | GSK wins EU nod to expand Nucala for COPD | 12 | Seeking Alpha | ||
| Fr | IN BRIEF: GSK Chair Jonathan Symonds buys GBP53,000 in shares | 9 | Alliance News | ||
| Fr | Director dealings: GSK chairman raises stake | 9 | Sharecast | ||
| Fr | EU regulator greenlights GSK lung disease therapy Nucala | 9 | Alliance News | ||
| Fr | GSK Wins EU Approval For Nucala In COPD Treatment | 291 | AFX News | WASHINGTON (dpa-AFX) - GSK plc (GSK) on Friday said the European Commission has approved Nucala as an add-on maintenance treatment for adults with uncontrolled chronic obstructive pulmonary... ► Artikel lesen | |
| Fr | EU gives GSK's Nucala green light for use in uncontrolled COPD | 3 | Sharecast | ||
| Fr | GSK PLC - Nucala COPD approved by the European Commission | 4 | RNS | ||
| Do | GSK PLC - Director/PDMR Shareholding | 17 | RNS | ||
| Mi | GSK oncology and HIV drugs leads to 7% sales rise in 2025 | 19 | Pharmaceutical Technology | ||
| Mi | As CEO, Luke Miels wants GSK to be more 'product-centric' | 11 | FiercePharma | ||
| Mi | GSK delivers in new CEO Miels' first financial update | 14 | pharmaphorum | ||
| Mi | GSK's new CEO looking for $2B to $4B deals 'hiding in plain sight' | 13 | FierceBiotech | ||
| Mi | GSK Stock Smashes 52-Week High On Strong Quarter, Dividend Hike | 41 | Benzinga.com | ||
| Mi | GSK Q4 2025 slides presentation: Specialty medicines drive earnings beat | 8 | Investing.com | ||
| Mi | LONDON MARKET MIDDAY: FTSE 100 strengthens as Beazley and GSK rise | 3 | Alliance News | ||
| Mi | GSK reaffirms profit growth projections for 2026 | 6 | Seeking Alpha | ||
| Mi | GSK: Besser als erwartet | 402 | Bernecker Börsenbriefe | Der Pharmakonzern konnte ein unerwartet starkes Schlussquartal vorweisen und damit besser abschneiden als gedacht. Der Umsatz von GSK klettert im vergangenen Jahr um 4 % auf knapp 32,7 Mrd. Pfund, währungsbereinigt... ► Artikel lesen | |
| Mi | GSK optimistic for 2026 after fourth-quarter results beat forecast | 12 | Alliance News | ||
| Mi | Pharmakonzern GSK schlägt sich besser als erwartet - Ziele bestätigt | 334 | dpa-AFX | LONDON (dpa-AFX) - Der Pharmakonzern GSK hat dank eines unerwartet starken Schlussspurts im abgelaufenen Jahr besser abgeschnitten als gedacht. "GSK hat 2025 erneut eine starke Leistung erzielt, die... ► Artikel lesen |
1 2 3 4 5 Weiter >>
575 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 45,800 | +2,20 % | Dax - Ausblick auf die neue Handelswoche: Bayer - Wann fällt die Marke von 50 Euro? | Nach einer überaus turbulenten Handelswoche richten sich die Blicke bereits auf die neue Handelswoche und damit auf den neuen Monat. Der Januar gestaltete sich für den Dax durchaus ambivalent. Dem Index... ► Artikel lesen | |
| NOVO NORDISK | 40,310 | +0,01 % | Novo Nordisk: Aktie wieder unter Druck - das müssen Anleger jetzt wissen | Die Aktie von Novo Nordisk hat seit ihrem Tief im November vergangenen Jahres eine starke Aufholjagd hingelegt. Zuletzt verlor das Papier allerdings an Schwung. Dabei ist der Titel auch unter eine wichtige... ► Artikel lesen | |
| PFIZER | 23,015 | -0,07 % | Ihre wichtigsten Termine: Frische Quartalszahlen von: Pepsico, PayPal, Pfizer, AMD, Merck & Co und KSB | © Foto: Jeff Chiu/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 07:00... ► Artikel lesen | |
| NOVARTIS | 132,06 | +0,03 % | Novartis Pharma AG: Novartis erzielte im Jahr 2025 eine Umsatzsteigerung im hohen einstelligen Prozentbereich, eine Kerngewinnmarge von 40% sowie weitere Fortschritte in der Pipeline | Ad-hoc-Mitteilung gemäss Art. 53 KR
Geschäftsjahr
Der Nettoumsatz wuchs um +8% (kWk1, +8% USD), das operative Kernergebnis1 verbesserte sich um +14% (kWk, +12% USD)
Das Umsatzwachstum... ► Artikel lesen | |
| MERCK KGAA | 121,45 | -0,49 % | BARCLAYS stuft MERCK KGAA auf 'Equal Weight' | LONDON (dpa-AFX Analyser) - Die britische Investmentbank Barclays hat die Einstufung für Merck KGaA mit einem Kursziel von 130 Euro auf "Equal Weight" belassen. Der Fokus liege auf möglichen Risiken... ► Artikel lesen | |
| GILEAD SCIENCES | 128,98 | -0,05 % | Gilead wins favorable U.S. label update for Yescarta | ||
| AURORA CANNABIS | 2,925 | -0,85 % | Aurora Cannabis Inc.: Aurora Cannabis Announces Fiscal 2026 Third Quarter Results | NASDAQ | TSX: ACB
Expands YoY Total Net Revenue1 by 7% to $94.2 million, with Global Medical Cannabis Net Revenue1 Increasing by 12% to a Record $76.2 million
... ► Artikel lesen | |
| SANOFI | 80,49 | +0,05 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| GSK | 25,300 | +1,12 % | Pharmakonzern GSK schlägt sich besser als erwartet - Ziele bestätigt | LONDON (dpa-AFX) - Der Pharmakonzern GSK hat dank eines unerwartet starken Schlussspurts im abgelaufenen Jahr besser abgeschnitten als gedacht. "GSK hat 2025 erneut eine starke Leistung erzielt, die... ► Artikel lesen | |
| ABBVIE | 189,20 | +0,11 % | Aktien New York Ausblick: Keine Tech-Erholung - Eli Lilly steigen kräftig | NEW YORK (dpa-AFX) - Mit Blick auf den Technologiesektor dürften sich die Investoren an den US-Börsen am Mittwoch zurückhalten. Der Nasdaq 100 Index, der am Vortag deutlich gefallen war, dürfte sich... ► Artikel lesen | |
| CANOPY GROWTH | 0,935 | -0,95 % | Canopy Growth: Treffer ohne Gewinn - Trading-Tipp wird zurückgezogen | ||
| ROCHE | 391,10 | -0,29 % | DZ BANK stuft ROCHE HOLDINGS AG auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Aktienwert für Roche von 324 auf 395 Franken angehoben und die Einstufung auf "Kaufen" belassen. "Die Pharma-Sparte hat den großen, nahezu parallelen... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 894,80 | -0,01 % | Abnehmpille schlägt ein: Starker Start ins neue Jahr - Novo Nordisk zeigt Eli Lilly die Rücklichter! | © Foto: Kristian Tuxen Ladegaard Berg - NurPhotoNach einem schwachen Vorjahr meldet sich Novo Nordisk 2026 eindrucksvoll zurück. Die neue Wegovy-Tablette sorgt in den USA für einen starken Marktstart.... ► Artikel lesen | |
| MERCK & CO | 103,20 | 0,00 % | Insider trades: Merck, Intel, Micron among notable names |
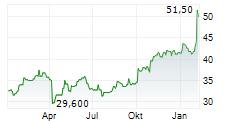
Das Unternehmen GSK PLC ADR kann der Branche Pharma zugeordnet werden. Mit einer aktuellen Kursveränderung von +0,50 (+0,98 %) liegt der Kurs bei 51,50 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GSK PLC ADR finden Sie auf Wallstreet Online.