- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 12.02. | Lyell Immunopharma, Inc: Lyell Immunopharma Announces Initiation of Patient Dosing in First-of-Its-Kind Phase 3 Head-To-Head CAR T-Cell Clinical Trial in Aggressive Large B-Cell Lymphoma | 866 | GlobeNewswire (Europe) | PiNACLE - H2H will evaluate the efficacy and safety of rondecabtagene autoleucel (ronde-cel) versus Investigator's choice of approved CD19 CAR T-cell therapies in patients with aggressive large B-cell... ► Artikel lesen | |
| 22.12.25 | Lyell Immunopharma pops on recent ASH data, immunotherapy expansion | 4 | Seeking Alpha | ||
| 07.12.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Presents New Clinical Data from Ongoing Trial of Ronde-Cel Showing High Rates of Durable Complete Responses in Patients with Large B-cell Lymphoma at the 67th ASH Annual Meeting and Exposition | 2 | GlobeNewswire (USA) | ||
| 05.12.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 13.11.25 | Lyell Immunopharma reports Q3 results | 3 | Seeking Alpha | ||
| 12.11.25 | Lyell Immunopharma, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 10.11.25 | Lyell Immunopharma buys exclusive global rights to colorectal cancer drug | 1 | Seeking Alpha | ||
| LYELL IMMUNOPHARMA Aktie jetzt für 0€ handeln | |||||
| 10.11.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Acquires Exclusive Global Rights to a Next-Generation CAR T-Cell Product Candidate in Clinical Development for Metastatic Colorectal Cancer | 145 | GlobeNewswire (Europe) | LYL273 has demonstrated a 67% overall response rate, an 83% disease control rate, and a manageable safety profile at the highest dose level studied to date in patients with refractory metastatic colorectal... ► Artikel lesen | |
| 10.11.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 03.11.25 | Lyell Immunopharma: Vielversprechende Daten zu dualer CAR-T-Zelltherapie bei Lymphomen | 1 | Investing.com Deutsch | ||
| 03.11.25 | Lyell Immunopharma, Inc: Lyell Announces Two Oral Presentations from the Phase 1/2 Clinical Trial of Ronde-Cel for the Treatment of Aggressive Large B-Cell Lymphoma at the 67th ASH Annual Meeting and Exposition | 2 | GlobeNewswire (USA) | ||
| 03.11.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 26.09.25 | Lucid Capital Markets initiates Lyell Immunopharma stock with Buy rating | 2 | Investing.com | ||
| 16.09.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 12.08.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Reports Business Highlights and Financial Results for the Second Quarter 2025 | 407 | GlobeNewswire (Europe) | Presented positive new clinical data demonstrating high rates of durable complete responses from the Phase 1/2 trial of LYL314 for the treatment of aggressive large B-cell lymphomaInitiated the PiNACLE... ► Artikel lesen | |
| 17.06.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Announces Positive New Clinical Data Demonstrating High Rates of Durable Complete Responses from the Phase 1/2 Trial of LYL314 for the Treatment of Aggressive Large B-cell Lymphoma | 230 | GlobeNewswire (Europe) | LYL314 demonstrated robust clinical responses, with an 88% overall response rate and a 72% complete response rate in patients treated in the third- or later-line setting (N = 25)71% of patients with... ► Artikel lesen | |
| 09.06.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Strengthens Clinical and Commercial Capabilities with Key Board and Executive Appointments | 267 | GlobeNewswire (Europe) | Mark J. Bachleda, PharmD, MBA appointed as independent member of the Board of DirectorsDavid Shook, MD appointed as Chief Medical Officer, Mark Meltz, JD as General Counsel and Corporate Secretary... ► Artikel lesen | |
| 13.05.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Reports Business Highlights and Financial Results for the First Quarter 2025 | 319 | GlobeNewswire (Europe) | Presenting new clinical data from Phase 1/2 multi-center clinical trial of LYL314, a next-generation dual-targeting CD19/CD20 CAR T-cell product candidate for the treatment of relapsed and/or refractory... ► Artikel lesen | |
| 15.04.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Receives Regenerative Medicine Advanced Therapy (RMAT) Designation for LYL314 for the Treatment of Relapsed and/or Refractory Large B-Cell Lymphoma | 256 | GlobeNewswire (Europe) | RMAT designation was granted based on promising clinical data from the ongoing Phase 1/2 trial of LYL314 in patients with relapsed and/or refractory large B-cell lymphoma.RMAT designation recognizes... ► Artikel lesen | |
| 11.03.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Reports Business Highlights and Financial Results for the Fourth Quarter and Full Year 2024 | 222 | GlobeNewswire (Europe) | Acquired ImmPACT Bio and strengthened clinical pipeline with the addition of IMPT-314, a next-generation dual-targeting CD19/CD20 CAR T-cell product candidate for the treatment of aggressive large... ► Artikel lesen |
20 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| EDITAS MEDICINE | 1,835 | -1,66 % | Editas Medicine, Inc.: Editas Medicine Nominates EDIT-401, an LDLR-Targeted Medicine, as Lead In Vivo Development Candidate | EDIT-401 achieved ~90% mean LDL-C reduction with single dose in non-human primates EDIT-401 on track for human proof-of-concept data by end of 2026 Strong cash position with operational runway into... ► Artikel lesen | |
| GALAPAGOS NV | 28,520 | -1,79 % | Galapagos NV: Galapagos Reports Full Year 2025 Financial Results and Provides Fourth Quarter Business Update | Strategic reset positions Galapagos for long-term value creation Robust 2025 year-end cash and financial investments of €3.0 billion and ongoing collaboration with Gilead enable flexibility to execute... ► Artikel lesen | |
| IMMATICS | 8,800 | +1,09 % | Immatics Presents IMA203CD8 PRAME Cell Therapy Data from Ongoing Dose Escalation and Shows Promising Initial Anti-tumor Activity in PRAME-Positive Tumors at ESMO-IO 2025 Congress | IMA203CD8 is a second-generation PRAME cell therapy with enhanced pharmacology in ongoing Phase 1a dose escalationManageable tolerability across all dose levelsEncouraging early clinical anti-tumor... ► Artikel lesen | |
| AUTOLUS THERAPEUTICS | 1,410 | -3,42 % | Cellares Corp: Autolus Therapeutics to Evaluate Automated Manufacturing of AUCATZYL (obe-cel) on the Cellares Cell ShuttleTM Platform | Autolus will evaluate Cellares' fully automated, high-throughput manufacturing platform in preparation for expansion into new indications
Autolus Therapeutics plc (Nasdaq: AUTL), a commercial-stage... ► Artikel lesen | |
| TANGO THERAPEUTICS | 11,140 | -2,37 % | Stifel reiterates Tango Therapeutics stock rating at Buy | ||
| TSCAN THERAPEUTICS | 1,060 | -4,93 % | TScan Therapeutics, Inc.: TScan Therapeutics Completes Enrollment in Cohort C of Phase 1 ALLOHA Trial and Announces FDA Clearance of Investigational New Drug Applications for Heme Candidates TSC-102-A01 and TSC-102-A03 | Completed enrollment of Cohort C in the Phase 1 ALLOHA trial; patients to be treated with commercial-ready manufacturing process Received FDA clearance of INDs for TSC-102-A01 and TSC-102-A03 targeting... ► Artikel lesen | |
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BIONTECH | 93,10 | -0,21 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen |
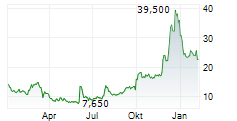
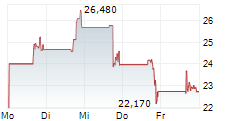
LYELL IMMUNOPHARMA INC gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell +4,85 % bei einem Aktienkurs von 24,230 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu LYELL IMMUNOPHARMA INC finden Sie auf Wallstreet Online.