| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 5,700 | 6,000 | 08:43 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| RYVU THERAPEUTICS Aktie jetzt für 0€ handeln |
0 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 87,00 | +0,29 % | BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen | |
| EVOTEC | 5,304 | 0,00 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| MEDIGENE | 0,039 | 0,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| PAION | 0,060 | -35,21 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,428 | -0,05 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| BIONXT SOLUTIONS | 0,375 | +1,35 % | BioNxt Solutions Inc.: BioNxt Announces Closing of Shares for Debt Settlement | VANCOUVER, BC / ACCESS Newswire / February 26, 2026 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTCQB:BNXTF)(FSE:BXT) is pleased to announce that, further to its news release dated... ► Artikel lesen | |
| DEFENCE THERAPEUTICS | 0,379 | -1,56 % | Defence Therapeutics Inc.: Defence Therapeutics to Showcase Accum Platform at Key International Industry Events in March | Montreal, Quebec--(Newsfile Corp. - March 2, 2026) - Defence Therapeutics Inc. (CSE: DTC) (FSE: DTC) (OTCQB: DTCFF) ("Defence" or the "Company"), a publicly traded biotechnology company advancing... ► Artikel lesen | |
| KUROS BIOSCIENCES | 29,100 | -1,42 % | Kuros Biosciences AG: Kuros Biosciences to provide investor update at Octavian and Baader Bank Conferences | Kuros Biosciences to provide investor updateat Octavian and Baader Bank Conferences
Schlieren (Zürich), Switzerland, January 13, 2026 - Kuros Biosciences ("Kuros" or the "Company") a leader in innovative... ► Artikel lesen | |
| NUREXONE BIOLOGIC | 0,410 | -0,24 % | Exosomen auf dem Prüfstand: NurExone legt den Grundstein für die erste klinische Studie am Menschen | ||
| HALOZYME THERAPEUTICS | 59,12 | -1,04 % | Halozyme skizziert strategische Wachstumspläne auf Gesundheitskonferenz von TD Cowen | ||
| IMMATICS | 8,295 | -2,01 % | Immatics Presents IMA203CD8 PRAME Cell Therapy Data from Ongoing Dose Escalation and Shows Promising Initial Anti-tumor Activity in PRAME-Positive Tumors at ESMO-IO 2025 Congress | IMA203CD8 is a second-generation PRAME cell therapy with enhanced pharmacology in ongoing Phase 1a dose escalationManageable tolerability across all dose levelsEncouraging early clinical anti-tumor... ► Artikel lesen | |
| BEONE MEDICINES LTD ADR | 250,00 | -2,34 % | BeOne Medicines Ltd.: BeOne Medicines präsentiert Finanzergebnisse für das vierte Quartal und das Gesamtjahr 2025 - weltweiter Erfolg von BRUKINSA und führende Rolle in der Onkologie | Weltweiter Umsatzerlös von 1,5 Milliarden US-Dollar und 5,3 Milliarden US-Dollar für das vierte Quartal und das Gesamtjahr, ein Anstieg von 33 bzw. 40 gegenüber den Vorjahresperioden Weltweiter... ► Artikel lesen | |
| SINO BIOPHARM | 0,603 | +0,97 % | SINO BIOPHARM (01177): VOLUNTARY ANNOUNCEMENT - EXCLUSIVE LICENSE AGREEMENT FOR ROVADICITINIB WITH SANOFI | ||
| ZAI LAB LTD ADR | 15,100 | -1,31 % | Zai Lab outlines 2026 transition year with multiple late-stage catalysts and targets first global approval by 2028 | ||
| CYBIN | 6,750 | -0,74 % | Helus Pharma Appoints Former Pfizer Chief Medical Officer Dr. Freda Lewis-Hall to Board of Directors & Chair of the Scientific Advisory Committee | In this role, Dr. Lewis-Hall will guide clinical development strategy, regulatory engagement, and translational rigor across Helus' novel serotonergic agonist ("NSA") portfolio This news release constitutes... ► Artikel lesen |
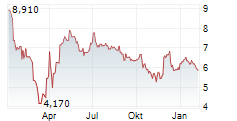
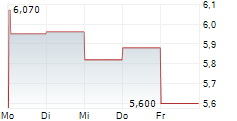
RYVU THERAPEUTICS SA gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 5,700 Euro und damit -4,04 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu RYVU THERAPEUTICS SA finden Sie auf Wallstreet Online.