| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 35,800 | 36,800 | 07:32 | |
| 35,800 | 36,800 | 07:31 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 19.02. | Earnings Summary: Spyre Therapeutics Q4 | 1 | Benzinga.com | ||
| 19.02. | Spyre Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 1 | SEC Filings | ||
| 19.02. | Spyre Therapeutics, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 19.02. | Spyre Therapeutics, Inc.: Spyre Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Corporate Update | 139 | GlobeNewswire (Europe) | On track for 6 proof-of-concept readouts in 2026 across the SKYLINE and SKYWAY Phase 2 trials Part A readouts from SKYLINE platform trial in ulcerative colitis ("UC") expected to begin in the second... ► Artikel lesen | |
| 19.02. | Spyre Therapeutics, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| 16.02. | Spyre Therapeutics (SYRE) Set POC Readout Priorities for 2026 | 3 | Insider Monkey | ||
| SPYRE THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 06.02. | Spyre Therapeutics, Inc.: Spyre Therapeutics Announces Grants of Inducement Awards | 3 | GlobeNewswire (USA) | ||
| 06.02. | Spyre Therapeutics appoints Kate Tansey Chevlen as Chief Commercial Officer | 2 | PMLiVE | ||
| 23.01. | Spyre Therapeutics, Inc.: Spyre Therapeutics Announces Grants of Inducement Awards | 1 | GlobeNewswire (USA) | ||
| 15.01. | Wall Street Bullish on Spyre Therapeutics, Inc. (SYRE) with Strong Buy Rating | 2 | Insider Monkey | ||
| 12.01. | Spyre Therapeutics accelerates clinical trial timeline for IBD treatments | 2 | Investing.com | ||
| 12.01. | Spyre Therapeutics, Inc.: Spyre Therapeutics Poised for Transformational 2026 With Six Expected Proof-of-Concept Readouts Beginning in Q2 | 201 | GlobeNewswire (Europe) | "6 in '26" expected proof-of-concept (POC) readouts across SKYLINE and SKYWAY trials SKYLINE platform trial in ulcerative colitis (UC) recruiting faster than expected with SPY001 enrollment complete... ► Artikel lesen | |
| 12.01. | Spyre Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 08.01. | Spyre Therapeutics, Inc.: Spyre Therapeutics Announces Grants of Inducement Awards | 1 | GlobeNewswire (USA) | ||
| 01.01. | Spyre Therapeutics: Biotech-Aktie mit Potenzial | 817 | sharedeals.de | Die Biotechnologiebranche ist geprägt von einem Spannungsfeld zwischen wissenschaftlicher Innovationskraft und der oft fragilen finanziellen Lage vieler Unternehmen. Umso bemerkenswerter sind Fälle... ► Artikel lesen | |
| 17.12.25 | Mizuho initiates Spyre Therapeutics stock with Outperform rating | 5 | Investing.com | ||
| 17.12.25 | Spyre Therapeutics: Mizuho startet Coverage mit "Outperform" und sieht 60 % Potenzial | 3 | Investing.com Deutsch | ||
| 05.12.25 | Spyre Therapeutics, Inc.: Spyre Therapeutics Announces Grants of Inducement Awards | 3 | GlobeNewswire (USA) | ||
| 01.12.25 | Spyre stock rating upgraded to Buy at Jones Trading on TL1A blockade potential | 3 | Investing.com | ||
| 07.11.25 | Spyre Therapeutics, Inc.: Spyre Therapeutics Announces Grants of Inducement Awards | 1 | GlobeNewswire (USA) |
1 2 3 Weiter >>
53 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| ORUKA THERAPEUTICS | 29,600 | +1,37 % | Oruka Therapeutics, Inc.: Oruka Therapeutics Reports Third Quarter 2025 Financial Results and Provides Corporate Update | ORKA-001 Phase 1 results presented at EADV show potential for once-per-year dosing, higher efficacy and off-treatment remission Over $500M cash and equivalents provides runway over one year past three... ► Artikel lesen | |
| PALVELLA THERAPEUTICS | 116,00 | +0,87 % | Palvella Therapeutics Inc.: Palvella Therapeutics Announces Positive Topline Results from Phase 3 SELVA Clinical Study of QTORIN 3.9% Rapamycin Anhydrous Gel (QTORIN rapamycin) in Microcystic Lymphatic Malformations | Primary endpoint met with statistically significant improvement (mean change of +2.13; p Achieved statistical significance on pre-specified key secondary endpoint (p 95% of trial participants aged... ► Artikel lesen | |
| DIANTHUS THERAPEUTICS | 47,000 | +0,43 % | Dianthus Therapeutics, Inc.: Leads Biolabs And Dianthus Therapeutics Announce Initiation of Phase 1 Trial Of LBL-047 (DNTH212) In Healthy Volunteers and Patients With Systemic Lupus Erythematosus (SLE) | LBL-047 (DNTH212) is a bifunctional fusion protein targeting plasmacytoid dendritic cell (pDC) BDCA2 to reduce Type 1 interferon production, while simultaneously inhibiting BAFF/APRIL to suppress... ► Artikel lesen | |
| LENZ THERAPEUTICS | 13,500 | -0,15 % | LENZ Therapeutics, Inc.: LENZ Therapeutics and Sarah Jessica Parker announce the launch of "Make it VIZZable", the VIZZ consumer campaign | Struggling to see up close? VIZZ is a revolutionary, once-daily eye drop and alternative to reading glasses to restore near vision for up to 10 hours The "Make it VIZZable" campaign features SJP enjoying... ► Artikel lesen | |
| QIAGEN | 41,960 | 0,00 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| EVOTEC | 5,880 | 0,00 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | -79,74 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | +24,33 % | Cardio Diagnostics Holdings, Inc. - 8-K, Current Report | ||
| ADMA BIOLOGICS | 15,580 | +2,70 % | ADMA Q4 EPS Up 14% Y/Y, Revenues Gain From Strong Asceniv Performance | ||
| RELAY THERAPEUTICS | 10,250 | +12,02 % | Relay Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| PRAXIS PRECISION MEDICINES | 336,75 | -1,05 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen |
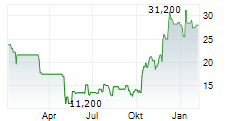
SPYRE THERAPEUTICS INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 37,400 Euro und damit +2,75 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SPYRE THERAPEUTICS INC finden Sie auf Wallstreet Online.