- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 24.02. | Artiva Biotherapeutics Names Thad Huston CFO | 3 | RTTNews | ||
| 24.02. | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Announces Appointment of Thad Huston as Chief Financial Officer and Award of Inducement Grant | 263 | GlobeNewswire (Europe) | SAN DIEGO, Feb. 24, 2026 (GLOBE NEWSWIRE) -- Artiva Biotherapeutics, Inc. (Nasdaq: ARTV) (Artiva), a clinical-stage biotechnology company whose mission is to develop effective, safe and accessible... ► Artikel lesen | |
| 19.02. | Artiva Biotherapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| ARTIVA BIOTHERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 21.01. | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Announces Upcoming Presentations at the 2026 Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR (Tandem Meetings) Highlighting Cost-Effectiveness of AlloNK in a Community Rheumatology ... | 1 | GlobeNewswire (USA) | ||
| 12.12.25 | Artiva Biotherapeutics, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 12.11.25 | Artiva BioTherapeutics GAAP EPS of -$0.88 | 3 | Seeking Alpha | ||
| 12.11.25 | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Announces Positive Initial Safety and Translational Data Supporting Deep B-Cell Depletion with AlloNK in Autoimmune Disease | 240 | GlobeNewswire (Europe) | 32 patients with autoimmune disease treated with AlloNK plus anti-CD20 monoclonal antibody (mAb) as of October 1, 2025, data cutoff All patients received AlloNK as outpatients, and the majority were... ► Artikel lesen | |
| 12.11.25 | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Reports Third Quarter 2025 Financial Results and Recent Business Highlights | 171 | GlobeNewswire (Europe) | Over 100 patients treated with AlloNK across autoimmune and oncology indications Refractory rheumatoid arthritis (RA) prioritized as lead indication following FDA Fast Track Designation for AlloNK®... ► Artikel lesen | |
| 12.11.25 | Artiva Biotherapeutics, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 12.11.25 | Artiva Biotherapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 17.10.25 | Cantor Fitzgerald reiterates Overweight rating on Artiva Biotherapeutics stock | 19 | Investing.com | ||
| 16.10.25 | Rheumatoide Arthritis: Artiva erhält FDA Fast-Track-Status für Zelltherapie AlloNK | 5 | Investing.com Deutsch | ||
| 16.10.25 | Artiva's AlloNK receives FDA Fast Track status for rheumatoid arthritis | 3 | Investing.com | ||
| 16.10.25 | Artiva Biotherapeutics stock soars after FDA Fast Track designation | 2 | Investing.com | ||
| 16.10.25 | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Announces Refractory Rheumatoid Arthritis as Lead Indication, Upcoming Data Releases, and Corporate Update | 405 | GlobeNewswire (Europe) | Prioritization of refractory rheumatoid arthritis (RA) as lead indication for AlloNK® development FDA Fast Track Designation received for AlloNK in refractory RA, representing the first drug candidate... ► Artikel lesen | |
| 13.05.25 | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Announces Longer-term Phase 1/2 Data Demonstrating Prolonged Durability for AlloNK in Combination with Rituximab in Patients with B-cell-Non-Hodgkin Lymphoma at the ASGCT ... | 220 | GlobeNewswire (Europe) | 64% (9/14) complete response rate with AlloNK + rituximab in heavily pretreated patients that were naïve to prior CAR-T cell therapy, in line with approved auto-CAR-T therapies in aggressive B-NHL... ► Artikel lesen | |
| 08.05.25 | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Reports First Quarter 2025 Financial Results, Recent Business Highlights | 390 | GlobeNewswire (Europe) | IND clearance and initiation of global basket trial exploring AlloNK® + rituximab in refractory rheumatoid arthritis, Sjögren's disease, idiopathic inflammatory myopathies and systemic sclerosis ... ► Artikel lesen | |
| 08.04.25 | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Appoints Subhashis Banerjee, M.D., as Chief Medical Officer | 858 | GlobeNewswire (Europe) | SAN DIEGO, April 08, 2025 (GLOBE NEWSWIRE) -- Artiva Biotherapeutics, Inc. (Nasdaq: ARTV), a clinical-stage biotechnology company whose mission is to develop effective, safe, and accessible cell therapies... ► Artikel lesen | |
| 24.03.25 | Artiva Biotherapeutics, Inc.: Artiva Biotherapeutics Reports Full Year 2024 Financial Results and Recent Business Highlights | 280 | GlobeNewswire (Europe) | Initial data for AlloNK® from autoimmune program expected H1 2025 Updated clinical data from Phase 1/2 trial exploring AlloNK + rituximab in NHL showing continued durability of response to be presented... ► Artikel lesen |
19 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 92,30 | -1,07 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| AMGEN | 329,30 | +0,23 % | AAPL, AA, BKR, and AMGN are among the top stocks for momentum, says Oppenheimer | ||
| NOVAVAX | 8,765 | +2,18 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| BIOGEN | 161,20 | -0,71 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| VIKING THERAPEUTICS | 28,845 | +0,58 % | Viking Therapeutics: The High-Stakes Weight Loss Contender | ||
| MAINZ BIOMED | 0,767 | +2,57 % | Mainz BioMed NV: Mainz Biomed to Present at the Pancreatic Cancer Session at Digestive Disease Week, Demonstrating Effectiveness of mRNA Biomarkers in Blood Samples | ||
| INTELLIA THERAPEUTICS | 13,110 | +12,44 % | Intellia Therapeutics, Inc.: Intellia Therapeutics Announces FDA Lift of Clinical Hold on MAGNITUDE Phase 3 Clinical Trial in ATTR-CM | CAMBRIDGE, Mass., March 02, 2026 (GLOBE NEWSWIRE) -- Intellia Therapeutics, Inc. (Nasdaq: NTLA), a leading biopharmaceutical company focused on revolutionizing medicine leveraging CRISPR gene editing... ► Artikel lesen | |
| TEMPUS AI | 44,800 | -0,88 % | Tempus AI Quartalszahlen: 90 % Umsatzplus bestätigen KI-Trend in der Medizin | CHICAGO, Illinois (IT-Times) - Das Diagnose-Technologie-Unternehmen Tempus AI hat seine Ergebnisse für das vierte Quartal 2025 veröffentlicht und ein rasantes Umsatzwachstum verzeichnet. Tempus AI -... ► Artikel lesen | |
| BIOCRYST PHARMACEUTICALS | 7,426 | +0,22 % | BioCryst Pharmaceuticals, Inc.: BioCryst Reports Full Year 2025 Financial Results and Provides Business Update | -Full year 2025 ORLADEYO® net revenue of $601.8 million (+38% y-o-y; +43% y-o-y excluding European ORLADEYO revenue following the sale of the European ORLADEYO business to Neopharmed Gentili S.p.A.... ► Artikel lesen | |
| BIOMARIN PHARMACEUTICAL | 51,62 | -1,19 % | FDA Expands BioMarin's PALYNZIQ Approval To Include Pediatric PKU Patients Ages 12 And Up | WASHINGTON (dpa-AFX) - BioMarin Pharmaceutical Inc. (BMRN) announced Friday that the U.S. Food and Drug Administration has approved the company's supplemental Biologics License Application for... ► Artikel lesen | |
| SAREPTA THERAPEUTICS | 14,000 | -1,27 % | Sarepta Therapeutics, Inc. - 10-K, Annual Report | ||
| EXELIXIS | 34,820 | -6,65 % | Forecasting The Future: 11 Analyst Projections For Exelixis | ||
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,436 | +0,70 % | PACIFIC BIOSCIENCES OF CALIFORNIA, INC. - S-8, Securities to be offered to employees in employee benefit plans | ||
| CARDIOL THERAPEUTICS | 0,860 | -0,81 % | Cardiol Therapeutics Inc.: Cardiol Therapeutics to Present at TD Cowen 46th Annual Health Care Conference | Toronto, Ontario--(Newsfile Corp. - February 25, 2026) - Cardiol Therapeutics Inc. (NASDAQ: CRDL) (TSX: CRDL) ("Cardiol" or the "Company"), a late-stage life sciences company focused on advancing... ► Artikel lesen | |
| VAXART | 0,510 | -19,05 % | Vaxart, Inc.: Vaxart Provides Business Update and Reports Third Quarter 2025 Financial Results | Entered into an exclusive license agreement with Dynavax for the Company's COVID-19 oral pill vaccine candidate for potential cumulative proceeds of up to $700 million plus royalties Completed enrollment... ► Artikel lesen |
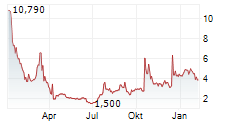
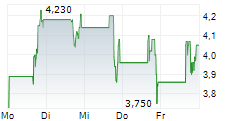
Das Unternehmen ARTIVA BIOTHERAPEUTICS INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell -1,03 % bei einem Aktienkurs von 5,770 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ARTIVA BIOTHERAPEUTICS INC finden Sie auf Wallstreet Online.