- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 19.02. | Biodexa Pharmaceuticals PLC: Biodexa Licenses Phase 1 Ready Drug Candidate from Otsuka for Rare Stomach Cancer | 906 | ACCESS Newswire | MTX240's mechanistic novelty may give it a long-awaited edge in treatments for gastrointestinal stromal tumors (GIST)Meg Flippin, Benzinga Staff Writer CARDIFF, UK / ACCESS Newswire / February 19, 2026... ► Artikel lesen | |
| 04.02. | Biodexa licenses Otsuka's molecular glue compound for GIST treatment | 4 | Investing.com | ||
| BIODEXA PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| 04.02. | Biodexa Pharmaceuticals Plc - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 04.02. | Biodexa Pharmaceuticals PLC: Biodexa Announces Exclusive License of Otsuka's OPB-171775, a potent Phase 1 ready Molecular Glue for GIST | 234 | GlobeNewswire (Europe) | February 4, 2026 Biodexa Announces Exclusive License of Otsuka's OPB-171775, a potent Phase 1 ready Molecular Glue for GIST Novel Mechanism of Action Shown to be Effective in TKI Resistant PDX Models... ► Artikel lesen | |
| 05.01. | Biodexa Promotes Fiona Sharp To CFO As Stephen Stamp Cedes Role To Become Only CEO | 3 | RTTNews | ||
| 05.01. | Biodexa pharmaceuticals appoints Fiona Sharp as CFO and board member | 1 | Investing.com | ||
| 05.01. | Biodexa Pharmaceuticals PLC: Appointment of Fiona Sharp to the Board as Chief Financial Officer and Company Secretary | 181 | GlobeNewswire (Europe) | January 05, 2026 Biodexa Pharmaceuticals PLC Appointment of Fiona Sharp to the Board as Chief Financial Officer and Company Secretary Biodexa Pharmaceuticals PLC ("Biodexa" or "the Company"), (Nasdaq:... ► Artikel lesen | |
| 05.01. | Biodexa Pharmaceuticals Plc - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 18.12.25 | Biodexa Pharmaceuticals: Aktie stürzt nach 10-Millionen-Dollar-Kapitalerhöhung ab | 6 | Investing.com Deutsch | ||
| 18.12.25 | Biodexa prices $10 million public offering to fund development programs | 2 | Investing.com | ||
| 18.12.25 | Biodexa prices $10 million public offering of units and warrants | 1 | Investing.com | ||
| 18.12.25 | Biodexa platziert öffentliches Angebot im Volumen von 10 Millionen US-Dollar | 1 | Investing.com Deutsch | ||
| 18.12.25 | Biodexa sichert sich 10 Millionen US-Dollar zur Finanzierung von Entwicklungsprogrammen | - | Investing.com Deutsch | ||
| 18.12.25 | Biodexa Pharmaceuticals prices $10M best-efforts public offering; shares down | 2 | Seeking Alpha | ||
| 18.12.25 | Biodexa Pharmaceuticals PLC: Biodexa Announces Pricing of $10 Million Public Offering | 205 | GlobeNewswire (Europe) | December 18, 2025 Biodexa Announces Pricing of $10 Million Public Offering Biodexa Pharmaceuticals PLC, (Nasdaq: BDRX) ("Biodexa" or the "Company"), a clinical stage biopharmaceutical company developing... ► Artikel lesen | |
| 18.12.25 | Biodexa Pharmaceuticals Plc - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 08.12.25 | Biodexa Pharmaceuticals Plc - F-1/A, Registration statement for certain foreign private issuers | 2 | SEC Filings | ||
| 01.12.25 | Biodexa enrolls first European patients in phase 3 FAP trial | 3 | Investing.com | ||
| 01.12.25 | Biodexa Pharmaceuticals PLC: Biodexa Announces Enrolment of First European Patients into Pivotal Phase 3 Serenta Trial in FAP | 251 | GlobeNewswire (Europe) | December 1, 2025 Biodexa Announces Enrolment of First European Patients into Pivotal Phase 3 Serenta Trial in FAP Biodexa Pharmaceuticals PLC ("Biodexa" or "the Company"), (Nasdaq: BDRX), a clinical... ► Artikel lesen | |
| 24.11.25 | Biodexa activates first European site for phase 3 FAP trial | 3 | Investing.com |
1 2 3 Weiter >>
55 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,610 | 0,00 % | MustGrow Biologics und die Partnerschaft mit Bayer: Darum steht die Aktie erst am Anfang | Die globale Landwirtschaft steht vor einem Paradox. Sie muss mehr Menschen ernähren, aber mit weniger chemischen Mitteln. Weltweit sind in 162 Ländern inzwischen 460 Pestizide verboten oder eingeschränkt.... ► Artikel lesen | |
| NOVO NORDISK | 32,040 | -0,40 % | maydornsmeinung: Silber, Nvidia, AMD, Microsoft, IBM, SAP, TeamViewer, Novo Nordisk, Nebius & Co. | In maydornsmeinung geht es zunächst um den Gesamtmarkt: Trotz Zoll-Chaos bleiben für Alfred die Unternehmensgewinne der entscheidende Faktor. Die Berichtssaison läuft stark, auch wenn sich die großen... ► Artikel lesen | |
| PFIZER | 23,280 | -0,11 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 142,00 | -0,73 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 126,25 | 0,00 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 128,00 | -0,26 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,175 | -0,47 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,31 | -0,26 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,930 | 0,00 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| ROCHE | 396,45 | -0,15 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,930 | +0,11 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 866,30 | -0,48 % | Eli Lilly und Novo Nordisk: Angst vor einem Preiskrieg bringt Aktien unter Druck | Novo Nordisk will ab 2027 die Preise für seine Blockbuster-Medikamente Wegovy und Ozempic in den USA drastisch senken und setzt damit Wettbewerber Eli Lilly unter Zugzwang. Die Aktien der beiden Pharmakonzerne... ► Artikel lesen | |
| ASTRAZENECA | 173,20 | -0,66 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TILRAY BRANDS | 6,550 | +0,61 % | MDMA-Herausforderer Bioxyne punktet mit 112% Umsatzplus. Und was macht Tilray? |
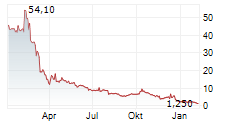
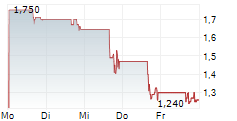
Das Unternehmen BIODEXA PHARMACEUTICALS PLC ADR kann der Branche Pharma zugeordnet werden. Der aktuelle Kurs liegt bei 1,010 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BIODEXA PHARMACEUTICALS PLC ADR finden Sie auf Wallstreet Online.