- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | Brookline Capital raises C4 Therapeutics stock price target on trial progress | 1 | Investing.com | ||
| Mo | C4 Therapeutics: Erster Patient in Phase-2-Studie zu Multiplem Myelom behandelt | 1 | Investing.com Deutsch | ||
| Mo | C4 Therapeutics, Inc.: C4 Therapeutics Announces First Patient Dosed in Phase 2 MOMENTUM Trial of Cemsidomide, an Oral IKZF1/3 Degrader, in Combination with Dexamethasone for Relapsed/Refractory Multiple Myeloma | 108 | GlobeNewswire (Europe) | Enrollment for Phase 2 MOMENTUM Trial Expected to Be Completed in Q1 2027 Phase 1b Trial of Cemsidomide in Combination with Elranatamab on Track to Initiate in Q2 2026 WATERTOWN, Mass., Feb. 23,... ► Artikel lesen | |
| Fr | Strong Analyst Confidence in C4 Therapeutics (CCCC)'s Cemsidomide Drives 270% Upside Potential | 1 | Insider Monkey | ||
| 09.02. | C4 Therapeutics, Inc.: C4 Therapeutics Announces Inducement Grant Under Nasdaq Listing Rule 5635(c)(4) | 1 | GlobeNewswire (USA) | ||
| 04.02. | C4 Therapeutics, Inc.: C4 Therapeutics to Participate in the Guggenheim Emerging Outlook: Biotech Summit 2026 | 1 | GlobeNewswire (USA) | ||
| 26.01. | C4 Therapeutics, Inc.: C4 Therapeutics Announces Inducement Grant Under Nasdaq Listing Rule 5635(c)(4) | 1 | GlobeNewswire (USA) | ||
| C4 THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 14.01. | C4 Therapeutics, Inc.: C4 Therapeutics Outlines Strategic Milestones to Advance Cemsidomide as a Potential Best-in-Class IKZF1/3 Degrader and Discovery Strategy Focused on Novel Targets in Clinically Validated Pathways | 366 | GlobeNewswire (Europe) | Cemsidomide Phase 2 MOMENTUM Trial On Track to Initiate in Q1 2026; Recommended Phase 2 Dose is 100 µg Cemsidomide Phase 1b Trial in Combination With Elranatamab On Track to Initiate in Q2 2026 Internal... ► Artikel lesen | |
| 14.01. | C4 Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 02.12.25 | C4 Therapeutics: TD Cowen startet Coverage mit Kaufempfehlung | 5 | Investing.com Deutsch | ||
| 02.12.25 | C4 Therapeutics stock initiated with Buy rating at TD Cowen | 3 | Investing.com | ||
| 21.11.25 | C4 Therapeutics files $400M mixed shelf offering | 3 | Seeking Alpha | ||
| 06.11.25 | C4 Therapeutics, Inc. - 10-Q, Quarterly Report | 2 | SEC Filings | ||
| 06.11.25 | C4 Therapeutics, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 16.10.25 | C4 Therapeutics prices $125M equity offering with warrants | 6 | Seeking Alpha | ||
| 16.10.25 | C4 Therapeutics, Inc.: C4 Therapeutics Announces Pricing of $125 Million Underwritten Offering | 5 | GlobeNewswire (USA) | ||
| 16.10.25 | C4 Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 01.10.25 | C4 Therapeutics, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 01.10.25 | C4 Therapeutics, Inc.: C4 Therapeutics Announces Clinical Trial Collaboration and Supply Agreement with Pfizer for the Combination of Cemsidomide and Elranatamab for the Treatment of Relapsed/Refractory Multiple Myeloma | 216 | GlobeNewswire (Europe) | WATERTOWN, Mass., Oct. 01, 2025 (GLOBE NEWSWIRE) -- C4 Therapeutics, Inc. (C4T) (Nasdaq: CCCC), a clinical-stage biopharmaceutical company dedicated to advancing targeted protein degradation science... ► Artikel lesen | |
| 23.09.25 | C4 Therapeutics stock price target raised to $10 by Wells Fargo on positive data | 10 | Investing.com |
1 2 Weiter >>
38 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIOGEN | 165,35 | -0,75 % | Biogen und BioArctic: Debatte um Alzheimer-Mittel in Deutschland spitzt sich zu | Im vergangenen Jahr hat Biogen mit seinem japanischen Partner Eisai das Alzheimer-Medikament Leqembi (Lecanemab) auch in Deutschland auf den Markt gebracht. Doch es herrschen Zweifel an der Wirksamkeit... ► Artikel lesen | |
| INOVIO PHARMACEUTICALS | 1,500 | -3,23 % | Is Inovio Pharmaceuticals Stock Going to $0? | ||
| OCUGEN | 1,409 | +1,59 % | Ocugen Appoints Rita Johnson-Greene to Chief Financial Officer | MALVERN, Pa., Feb. 09, 2026 (GLOBE NEWSWIRE) -- Ocugen, Inc. (Ocugen or the Company) (NASDAQ: OCGN), a pioneering biotechnology leader in gene therapies for blindness diseases, today announced the... ► Artikel lesen | |
| VIKING THERAPEUTICS | 29,005 | -0,75 % | Viking Therapeutics Stock Gains Amid Obesity Drug Buzz | ||
| TEMPUS AI | 49,200 | +1,23 % | What's Next: Tempus AI's Earnings Preview | ||
| ABIVAX | 111,20 | -0,71 % | Abivax-Aktie: Neue Gerüchte und ein steigender Kurs... | Kaum ein anderer Biotech-Titel steht derzeit so sehr im Fokus wie die Aktie von Abivax. Seit Monaten kursieren Übernahmegerüchte rund um das französische Unternehmen. Ein aktueller Auftritt auf dem... ► Artikel lesen | |
| XOMA ROYALTY | 21,000 | -2,78 % | XOMA Royalty Corporation: XOMA Royalty Announces Closing of Tender Offer and Completed Acquisition of Generation Bio, Inc. | EMERYVILLE, Calif., Feb. 09, 2026 (GLOBE NEWSWIRE) -- XOMA Royalty Corporation (NASDAQ: XOMA) ("XOMA Royalty" or the "Company"), a biotechnology royalty aggregator playing a distinctive role in helping... ► Artikel lesen | |
| CELLDEX THERAPEUTICS | 20,600 | -0,96 % | Celldex Therapeutics, Inc.: Celldex Announces Multiple Upcoming Presentations at AAAAI 2026 Supporting Barzolvolimab's First-in-Class and Best-in-Disease Profile | ||
| CARDIFF ONCOLOGY | 1,308 | -2,10 % | Cardiff Oncology, Inc.: Cardiff Oncology Announces Positive Update from its Randomized Phase 2 Trial of Onvansertib in First-line RAS-mutated mCRC | - Onvansertib added to FOLFIRI/bev first-line standard of care regimen showed dose-dependent improvement in overall response rates and durability trends as measured by progression-free survival in... ► Artikel lesen | |
| GUARDANT HEALTH | 81,28 | +1,32 % | Guardant Health buys Israeli co MetaSight Diagnostics | ||
| COHERUS ONCOLOGY | 1,380 | +0,11 % | Coherus closes $50.1 million public offering of common stock | ||
| AC IMMUNE | 2,195 | +2,57 % | J&J stoppt Rekrutierung in Alzheimer-Studie mit AC Immune | ||
| AGENUS | 2,760 | +6,15 % | Agenus Inc.: Agenus meldet Abschluss einer strategischen Kooperationsvereinbarung mit Zydus Lifesciences im Wert von 141 Millionen US-Dollar zur Weiterentwicklung von BOT+BAL und Stärkung der Produktionskapazitäten in den USA | Agenus Inc. (Nasdaq: AGEN), ein führendes Unternehmen auf dem Gebiet der Immunonkologie, gab heute den Abschluss seiner bereits angekündigten strategischen Zusammenarbeit mit Zydus Lifesciences... ► Artikel lesen | |
| ARDELYX | 5,206 | +6,83 % | Ardelyx, Inc.: Ardelyx Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | IBSRELA revenue grew 73% in 2025 to $274.2 million and total revenues reached $407.3 million Patient-first XPHOZAH strategy preserved access and drove growth in total dispenses Development programs... ► Artikel lesen | |
| ARCTURUS THERAPEUTICS | 7,670 | 0,00 % | Arcturus Therapeutics Provides Interim Phase 2 Data for Cystic Fibrosis Program | ARCT-032 generally safe and well tolerated
Meaningful trends of clinical activity observed via high resolution CT scans
After only 28 days of treatment with ARCT-032 (10 mg), 4 out of 6 Class... ► Artikel lesen |
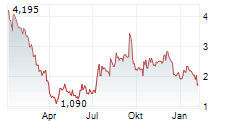
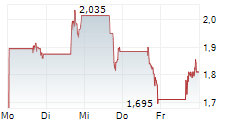
C4 THERAPEUTICS INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 2,315 Euro und damit +7,18 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu C4 THERAPEUTICS INC finden Sie auf Wallstreet Online.