| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
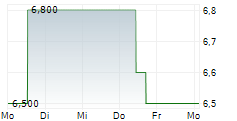
| 7,500 | 7,600 | 18:39 | |
| 7,500 | 7,650 | 18:31 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 17:26 | Cullgen ditches reverse merger with Pulmatrix to go with Gyre in $300M buyout | 3 | FierceBiotech | ||
| 15:24 | GYRE THERAPEUTICS, INC. - 8-K, Current Report | 1 | SEC Filings | ||
| 05.01. | Zulassungsfortschritt in China: Aktie von Gyre Therapeutics legt deutlich zu | 3 | Investing.com Deutsch | ||
| 05.01. | Gyre Pharmaceuticals treibt Zulassung für Leberfibrose-Medikament in China voran | 6 | Investing.com Deutsch | ||
| 05.01. | Gyre Pharmaceuticals advances liver fibrosis drug toward China approval | 1 | Investing.com | ||
| GYRE THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 05.01. | GYRE THERAPEUTICS, INC. - 8-K, Current Report | - | SEC Filings | ||
| 07.11.25 | GYRE THERAPEUTICS, INC. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 07.11.25 | Gyre Therapeutics GAAP EPS of $0.03 in-line, revenue of $30.56M misses by $2.37M | 6 | Seeking Alpha | ||
| 07.11.25 | GYRE THERAPEUTICS, INC. Announces Advance In Q3 Income | - | RTTNews | ||
| 07.11.25 | Gyre Therapeutics Inc.: Gyre Therapeutics Reports Third Quarter 2025 and Year-to-Date Financial Results and Provides Business Update | 231 | GlobeNewswire (Europe) | Net income of $5.9 million and $11.2 million for the three and nine months ended September 30, 2025, respectively Full-year revenue guidance revised to $115-118 million (from $118 - $128 million... ► Artikel lesen | |
| 07.11.25 | GYRE THERAPEUTICS, INC. - 8-K, Current Report | - | SEC Filings | ||
| 16.10.25 | H.C. Wainwright reiterates Buy rating on Gyre Therapeutics stock at $18 | 4 | Investing.com | ||
| 16.10.25 | H.C. Wainwright bestätigt Kaufempfehlung für Gyre Therapeutics mit Kursziel 18 $ | 3 | Investing.com Deutsch | ||
| 15.10.25 | Gyre Therapeutics completes enrollment for pneumoconiosis drug trial | 1 | Investing.com | ||
| 15.10.25 | Gyre Therapeutics Inc.: Gyre Therapeutics Announces Completion of Patient Enrollment in Phase 3 Clinical Trial of Pirfenidone Capsules for the Treatment of Pneumoconiosis | 288 | GlobeNewswire (Europe) | SAN DIEGO, Oct. 15, 2025 (GLOBE NEWSWIRE) -- Gyre Therapeutics (Nasdaq: GYRE), an innovative, commercial-stage biopharmaceutical company dedicated to advancing fibrosis-first therapies across organ... ► Artikel lesen | |
| 14.10.25 | Gyre to present phase 3 hydronidone liver fibrosis trial results | 3 | Investing.com | ||
| 14.10.25 | Gyre Therapeutics Inc.: Gyre Therapeutics to Present Results from Positive Phase 3 Clinical Trial Evaluating Hydronidone for the Treatment of Liver Fibrosis in Chronic Hepatitis B at AASLD-The Liver Meeting 2025 | 2 | GlobeNewswire (USA) | ||
| 10.10.25 | Jefferies initiates Gyre Therapeutics stock with Buy rating, $16 target | 1 | Investing.com | ||
| 10.09.25 | GYRE THERAPEUTICS, INC. - 8-K, Current Report | 2 | SEC Filings | ||
| 11.08.25 | Gyre Therapeutics Inc.: Gyre Therapeutics Reports Second Quarter 2025 and Year-to-Date Financial Results and Provides Business and Leadership Update | 253 | GlobeNewswire (Europe) | Net income of $1.6 million and $5.3 million for the three and six months ended June 30, 2025, respectively; reaffirms full-year revenue guidance of $118-128 million Ping Zhang, Executive... ► Artikel lesen |
1 2 Weiter >>
23 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,60 | +0,32 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,686 | -2,94 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BB BIOTECH | 52,10 | +1,36 % | BB Biotech: Von Innovation zu Wertrealisierung - Der Biotech-Markt in einer neuen Phase | Nach mehreren Jahren, die von erhöhten Kapitalkosten, Bewertungsdruck und ausgeprägter Risikoaversion geprägt waren, hat sich das Umfeld seit dem vergangenen Jahr spürbar stabilisiert. Finanzierungsbedingungen... ► Artikel lesen | |
| MEDIGENE | 0,036 | -10,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,295 | -0,23 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| MODERNA | 46,325 | +2,16 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PAION | 0,070 | -22,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,746 | +0,34 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| AMGEN | 330,20 | +0,50 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,300 | +23,81 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,691 | +1,32 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| BIOGEN | 159,35 | -1,85 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,690 | +0,37 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht | ||
| ILLUMINA | 111,54 | -2,00 % | Evercore ISI reiterates Illumina stock rating on competitive positioning |
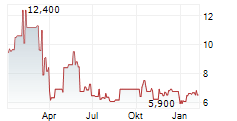
Das Unternehmen GYRE THERAPEUTICS INC kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 7,250 Euro und damit +3,57 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GYRE THERAPEUTICS INC finden Sie auf Wallstreet Online.