- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 13.02. | Citius Pharmaceuticals Q1 Earnings Report: What Investors Need to Know | 10 | Benzinga.com | ||
| 13.02. | Citius Pharmaceuticals, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 13.02. | Citius Pharmaceuticals, Inc. - 10-Q, Quarterly Report | 2 | SEC Filings | ||
| CITIUS PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| 13.02. | Citius Pharmaceuticals, Inc. Announces First Reported Revenue Following Successful Launch of LYMPHIR | 188 | PR Newswire | Company reports $3.9 million in revenue generated from initial sales in December 2025 and provides first fiscal quarter 2026 financial results
CRANFORD, N.J., Feb.... ► Artikel lesen | |
| 28.01. | Citius Pharmaceuticals, Inc. - 10-K/A, Annual Report | 3 | SEC Filings | ||
| 24.12.25 | Citius Pharmaceuticals (CTXR) Surges 22% After Hours - Here's Why | 12 | Benzinga.com | ||
| 24.12.25 | Citius Pharmaceuticals Inc. Full Year Loss Decreases | 3 | RTTNews | ||
| 23.12.25 | Citius Pharma launches cancer immunotherapy LYMPHIR in the U.S. | 1 | Investing.com | ||
| 23.12.25 | Citius Pharmaceuticals GAAP EPS of -$3.38 misses by $0.25 | 1 | Seeking Alpha | ||
| 23.12.25 | Citius Pharmaceuticals GAAP EPS of -$3.38 | 1 | Seeking Alpha | ||
| 23.12.25 | Citius Pharmaceuticals, Inc. Reports Fiscal Year 2025 Financial Results and Provides Business Update | 392 | PR Newswire | Subsidiary, Citius Oncology, launches cancer immunotherapy, LYMPHIR, in the U.S. in December 2025
CRANFORD, N.J., Dec. 23, 2025 /PRNewswire/ -- Citius Pharmaceuticals... ► Artikel lesen | |
| 23.12.25 | Citius Pharmaceuticals, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 23.12.25 | Citius Pharmaceuticals, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| 01.12.25 | Citius Pharmaceuticals, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 21.10.25 | Citius Pharmaceuticals closes $6 million registered direct offering | 6 | Investing.com | ||
| 21.10.25 | Citius Pharmaceuticals, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 12.08.25 | Citius Pharmaceuticals, Inc. Reports Fiscal Third Quarter 2025 Financial Results and Provides Business Update | 307 | PR Newswire | $12.5 million in gross financings raised during the quarter, with an additional $9 million raised by Citius Oncology in July 2025, to facilitate LYMPHIR pre-launch... ► Artikel lesen | |
| 08.07.25 | Citius Pharmaceuticals, Inc.: Citius Pharmaceuticals Regains Compliance with Nasdaq Minimum Bid Price Requirement | 302 | PR Newswire | CRANFORD, N.J., July 8, 2025 /PRNewswire/ -- Citius Pharmaceuticals, Inc. ("Citius Pharma" or the "Company") (Nasdaq: CTXR) today announced that it has received... ► Artikel lesen | |
| 14.05.25 | Citius Pharmaceuticals, Inc. Reports Fiscal Second Quarter 2025 Financial Results and Provides Business Update | 308 | PR Newswire | CRANFORD, N.J., May 14, 2025 /PRNewswire/ -- Citius Pharmaceuticals, Inc. ("Citius Pharma" or the "Company") (Nasdaq: CTXR), a biopharmaceutical company dedicated... ► Artikel lesen |
19 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,610 | -2,95 % | Aktien von Rheinmetall, Infineon, Bayer & Co. sind es nicht! ... und wie Börsenweisheiten über das schwierige Jahr 2025 hinweghelfen konnten. | Guten Tag, liebe Leserinnen und Leser,
viele von Ihnen kennen sie wahrscheinlich gar nicht mal. Aber vielleicht ist Ihnen doch schon die ein oder andere Börsenweisheit untergekommen, die aufgrund ihrer... ► Artikel lesen | |
| NOVO NORDISK | 32,065 | +1,23 % | Novo Nordisk schockt Anleger: Abnehmmittel-Studie enttäuscht - Aktie bricht ein, während Eli Lilly zulegt | Ein Dämpfer für den dänischen Pharmariesen: Die jüngste Studie zum Hoffnungsträger Cagrisema verfehlt die Erwartungen deutlich. Anleger reagieren geschockt. Lachender Dritter ist Eli Lilly. So prognostiziert... ► Artikel lesen | |
| PFIZER | 23,240 | -0,64 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| NOVARTIS | 142,78 | +0,45 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 126,25 | +0,48 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 127,98 | +1,51 % | Gilead Sciences schlägt zu - 68 Prozent Aufschlag | Die US-amerikanische Biotech-Gesellschaft Gilead Sciences will sich im Bereich der Onkologie verstärken. Mit der geplanten Übernahme von Arcellx erwirbt das Unternehmen die volle Kontrolle über eine... ► Artikel lesen | |
| AURORA CANNABIS | 3,205 | -2,29 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,51 | -0,28 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,930 | +0,48 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 199,40 | +1,42 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| CANOPY GROWTH | 0,936 | -0,64 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| ELI LILLY | 880,00 | -1,22 % | Up Over 400% in 5 Years, Is It Too Late to Invest in Eli Lilly Stock? | ||
| MERCK & CO | 104,00 | -0,76 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen | |
| ASTRAZENECA | 174,20 | -1,28 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TILRAY BRANDS | 6,500 | -2,69 % | MDMA-Herausforderer Bioxyne punktet mit 112% Umsatzplus. Und was macht Tilray? |
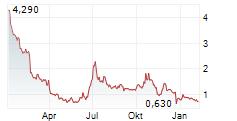
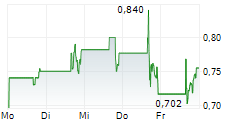
Das Unternehmen CITIUS PHARMACEUTICALS INC kann der Branche Pharma zugeordnet werden. Die relative Kursveränderung beträgt aktuell -3,81 % bei einem Aktienkurs von 0,718 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CITIUS PHARMACEUTICALS INC finden Sie auf Wallstreet Online.