| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 2,040 | 2,085 | 09:24 | |
| 2,020 | 2,070 | 09:17 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 02.02. | CROSSJECT announces initiation of coverage of its stock by Portzamparc (BNP Paribas Group) | 619 | GlobeNewswire (Europe) | CROSSJECT announces initiation of coverage of its stock by Portzamparc (BNP Paribas Group) DIJON, France - February 2, 2026 (7:15 a.m. CET) - CROSSJECT (ISIN: FR0011716265; Euronext: ALCJ), the specialty... ► Artikel lesen | |
| CROSSJECT Aktie jetzt für 0€ handeln | |||||
| 14.11.25 | CROSSJECT completes €5 million financing with Vatel Capital | 597 | GlobeNewswire (Europe) | Press release CROSSJECT completes €5 million financing with Vatel Capital DIJON, France - November 14th, 2025 (8.30 PM CET) - CROSSJECT (ISIN: FR0011716265; Euronext: ALCJ), a specialty pharmaceuticals... ► Artikel lesen | |
| 24.09.25 | CROSSJECT presents its financial results and key highlights for the first half of 2025 | 299 | GlobeNewswire (Europe) | Press release CROSSJECT presents its financial results and key highlights for the first half of 2025 Cash position: €6.3 million, compared to €7.0 million as of December 31 2024, demonstrating the... ► Artikel lesen | |
| 22.09.25 | CROSSJECT Secures Additional BARDA Funding for the Progressive Developmental and FDA Authorization of the ZEPIZURE (ZENEO Midazolam) | 195 | GlobeNewswire (Europe) | Press release CROSSJECT Secures Additional BARDA Funding for the Progressive Developmental and FDA Authorization of the ZEPIZURE® (ZENEO® Midazolam) DIJON, France - September 22, 2025 (7.30 AM... ► Artikel lesen | |
| 11.06.25 | CROSSJECT advances on the development of ZEPIZURE Junior, its solution for epilepsy crises in children | 231 | GlobeNewswire (Europe) | Press release CROSSJECT advances on the development of ZEPIZURE® Junior, its solution for epilepsy crises in children Announces the effective calibration of ZENEO® for the use of ZEPIZURE® in the... ► Artikel lesen | |
| 03.06.25 | Maxim Group initiates the coverage of the CROSSJECT share with a BUY recommendation and a target price of €4.00 | 168 | GlobeNewswire (Europe) | Press release Maxim Group initiates the coverage of the CROSSJECT share with a BUY recommendation and a target price of €4.00 Dijon, France 03 June 2025 (07.30 CET) -- CROSSJECT (ISIN: FR0011716265... ► Artikel lesen | |
| 15.05.25 | CROSSJECT's ZENEO needle-free autoinjector consistently matches depth of traditional intramuscular injections and exceeds the standards needle length of currently utilized auto-injectors | 287 | GlobeNewswire (Europe) | Press Release CROSSJECT's ZENEO® needle-free auto-injector consistently matches depth of traditional intramuscular injections and exceeds the standards needle length of currently utilized auto-injectors... ► Artikel lesen | |
| 27.03.25 | CROSSJECT reports financial results for 2024 | 310 | GlobeNewswire (Europe) | Press Release CROSSJECT reports financial results for 2024 Continued satisfactory production of regulatory batches with a view to filing in Q2 2025.Recruitment of Tony Tipton as Chief Operating Officer... ► Artikel lesen | |
| 25.03.25 | CROSSJECT updates on its collaboration with ETON PHARMACEUTICALS, Inc. and the ZENEO adrenal insufficiency program | 412 | GlobeNewswire (Europe) | Press Release CROSSJECT updates on its collaboration with ETON PHARMACEUTICALS, Inc. and the ZENEO® adrenal insufficiency program CROSSJECT enhanced its collaboration with Eton Pharmaceuticals,... ► Artikel lesen |
9 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| FRESENIUS | 49,930 | -2,71 % | Fresenius: Weiteres Wachstum in Sicht - Aktie bärenstark | Am kommenden Mittwoch (25. Februar) wird der Gesundheitskonzern Fresenius die Zahlen für das vergangene vierte Quartal respektive Gesamtjahr 2025 vorlegen. Der Markt rechnet sowohl umsatz- als auch... ► Artikel lesen | |
| SIEMENS HEALTHINEERS | 41,090 | -3,07 % | RBC stuft Siemens Healthineers auf 'Outperform' | NEW YORK (dpa-AFX Analyser) - Die kanadische Bank RBC hat Siemens Healthineers mit einem Kursziel von 55 Euro auf "Outperform" belassen. Die Nervosität der Anleger vor einem möglichen Verkauf des Diagnostikgeschäfts... ► Artikel lesen | |
| GERRESHEIMER | 16,040 | +2,17 % | DAX fester, Bitcoin tiefer: FMC, MTU, IBM, Telekom, Infineon, Airbus, SAP, Gerresheimer im ... | Der DAX hat am Montag Verluste einstecken müssen. Aus dem Handel ging er mit einem Minus von 1,1 Prozent bei 24.991,97 Punkten. Der Start in den Dienstag dürfte hingegen etwas freundlicher sein. Der... ► Artikel lesen | |
| DRAEGERWERK | 91,00 | +1,68 % | Draegerwerk AG & Co. KGaA: Zollentlastung bereits eingepreist, Aufwärtspotenzial wird kleiner, Kursziel erhöht; auf HALTEN gestuft. | Die Zollerlassungen in den USA bieten einen inkrementellen Unterstützungseffekt für die Draegerwerk, jedoch wird der Nutzen voraussichtlich schrittweise erfolgen. Während bestimmte Zölle aufgehoben... ► Artikel lesen | |
| INTUITIVE SURGICAL | 425,70 | -0,06 % | Im Krankenhaus auf Aktiensuche: Paul Hartmann, Technogym und Intuitive Surgical | Ein paar Dinge habe ich nach meinen Krankenhaus-Aufenthalten im November, Dezember und Januar gelernt...
...zum Beispiel: Es ist egal, wie schlecht es dir geht, es geht immer noch jemandem schlechter... ► Artikel lesen | |
| SERNOVA BIOTHERAPEUTICS | 0,088 | +2,92 % | Sernova Biotherapeutics: Sernova Biotherapeutics Announces Warrant Amendments | Toronto, Ontario and Boston, Massachusetts--(Newsfile Corp. - February 20, 2026) - Sernova Biotherapeutics (TSX: SVA) (OTC: SEOVF) (FSE: PSH0) ("the Company"), a leading regenerative medicine company... ► Artikel lesen | |
| FAMICORD | 5,100 | +0,99 % | EQS-News: FamiCord AG beschleunigt profitables Wachstum im dritten Quartal 2025 und stärkt Qualität und Visibilität der Umsatzentwicklung | EQS-News: FamiCord AG
/ Schlagwort(e): 9-Monatszahlen
FamiCord AG beschleunigt profitables Wachstum im dritten Quartal 2025 und stärkt Qualität und Visibilität der Umsatzentwicklung... ► Artikel lesen | |
| GERATHERM MEDICAL | 2,640 | -1,49 % | GERATHERM MEDICAL AG - Schwäche mit Einordnung | ||
| ALIGN TECHNOLOGY | 158,45 | -1,52 % | ALIGN TECHNOLOGY INC - 8-K, Current Report | ||
| NUGEN MEDICAL DEVICES | 0,019 | +3,30 % | NuGen Medical Devices Inc.: NuGen Medical Devices Inc. Provides Update on Health Canada License | Toronto, Ontario--(Newsfile Corp. - February 5, 2026) - NuGen Medical Devices Inc. (TSXV: NGMD) ("NuGen" or the "Company") is initiating a temporary halt on its devices in Canada due to the lapse... ► Artikel lesen | |
| PLUS THERAPEUTICS | 0,303 | -6,72 % | Plus Therapeutics Inc.: Plus Therapeutics Announces New Category III CPT Code for Convection-Enhanced Delivery Used with REYOBIQ | HOUSTON, Feb. 25, 2026 (GLOBE NEWSWIRE) -- Plus Therapeutics, Inc. (NASDAQ: PSTV) (the "Company"), a healthcare company developing and commercializing precision diagnostics and radiopharmaceuticals... ► Artikel lesen | |
| OTTOBOCK | 56,40 | +0,89 % | Ottobock: Verkauf geplant - die Details | Der Prothesenhersteller Ottobock will sich von einem weiteren Geschäftsbereich trennen. Wie zu Wochenstart bekannt wurde, strebt das Unternehmen, das erst im vergangenen Jahr an die Frankfurter Wertpapierbörse... ► Artikel lesen | |
| CARDINAL HEALTH | 192,05 | -0,98 % | Cardinal Health report highlights cost savings and provider confidence in biosimilars | New analysis shows biosimilars are projected to strengthen cost savings and provider confidence across the healthcare ecosystem, with oncology leading in adoption... ► Artikel lesen | |
| RESMED | 216,00 | -0,37 % | ResMed At $250: Missed Opportunity Or A Trap In The Making? | ||
| ALIGNMENT HEALTHCARE | 19,220 | -5,83 % | Alignment Healthcare Reports Fourth Quarter and Full-Year 2025 Results; Beats High-End of Guidance Across All Key Metrics | Delivers full-year revenue of $3.95 billion, representing 46.1% growth year-over-year Exceeds high-end of fourth quarter and full-year guidance across all key metrics: membership, revenue, adjusted... ► Artikel lesen |
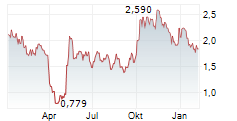
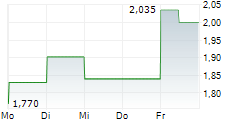
CROSSJECT SA gehört der Branche Gesundheitswesen an. Der aktuelle Kurs liegt bei 1,880 Euro und damit -4,95 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CROSSJECT SA finden Sie auf Wallstreet Online.