- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | Entrada Therapeutics, Inc.: Entrada Therapeutics Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 1 | GlobeNewswire (USA) | ||
| Do | Entrada Therapeutics: EPS übertrifft Schätzungen um 0,04 $ - Umsatz schlechter als erwartet | 2 | Investing.com Deutsch | ||
| Do | Entrada Therapeutics GAAP EPS of -$0.94 beats by $0.30, revenue of $1.3M misses by $6.11M | 1 | Seeking Alpha | ||
| Do | Entrada Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 2 | SEC Filings | ||
| ENTRADA THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| Do | Earnings Summary: Entrada Therapeutics Q4 | 1 | Benzinga.com | ||
| Do | Entrada Therapeutics, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| Do | Entrada Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 08.01. | Entrada Therapeutics legt Zeitplan für wichtige DMD-Studiendaten vor | 3 | Investing.com Deutsch | ||
| 08.01. | Entrada Therapeutics, Inc.: Entrada Therapeutics Highlights Progress Across its Portfolio of RNA-based Therapeutics for the Treatment of Neuromuscular and Ocular Diseases | 177 | GlobeNewswire (Europe) | -- Company on track to report ELEVATE-44-201 data from the first cohort in Q2 2026 and ELEVATE-45-201 data from the first cohort in mid-2026 - -- Expects to initiate global Phase 1/2 MAD clinical... ► Artikel lesen | |
| 08.01. | Entrada Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 04.12.25 | Entrada Therapeutics, Inc.: Entrada Therapeutics Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 2 | GlobeNewswire (USA) | ||
| 06.11.25 | Entrada Therapeutics: EPS verfehlt Schätzungen um 0,07 $ - Umsatz schlechter als erwartet | 2 | Investing.com Deutsch | ||
| 06.11.25 | Entrada Therapeutics GAAP EPS of -$1.06, revenue of $1.6M | 2 | Seeking Alpha | ||
| 06.11.25 | Entrada Therapeutics, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 06.11.25 | Entrada Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 17.09.25 | Goldman Sachs initiates coverage on Entrada Therapeutics stock with Early-Stage Biotech rating | 2 | Investing.com | ||
| 05.09.25 | Entrada awards $100,000 in grants to Duchenne muscular dystrophy groups | 2 | Investing.com | ||
| 05.09.25 | Entrada Therapeutics vergibt Fördermittel von 100.000 US-Dollar an Duchenne-Organisationen | 1 | Investing.com Deutsch | ||
| 05.09.25 | Entrada Therapeutics, Inc.: Entrada Therapeutics Announces Recipients of Third Annual DREAMS Grant Program | 2 | GlobeNewswire (USA) | ||
| 02.09.25 | Entrada Therapeutics, Inc.: Entrada Therapeutics Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 218 | GlobeNewswire (Europe) | BOSTON, Sept. 02, 2025 (GLOBE NEWSWIRE) -- Entrada Therapeutics, Inc. (Nasdaq: TRDA) today announced that the Company granted an aggregate of 54,360 restricted stock units ("RSUs") and options to... ► Artikel lesen |
1 2 Weiter >>
24 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 89,65 | -2,61 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,312 | -6,58 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BB BIOTECH | 51,00 | -1,16 % | BB Biotech: Gleich mehrere Zukäufe - spannende News erwartet | Im Schlussquartal 2025 hat die Schweizer Biotechgesellschaft BB Biotech einige Veränderungen am Portfolio vorgenommen. Bereits kurz nach dem Kauf wurde bei zwei Werten bereits eine Übernahme angekündigt:... ► Artikel lesen | |
| MEDIGENE | 0,035 | -13,73 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,120 | -0,42 % | Shortseller-Positionen aktuell: freenet, GFT, HelloFresh, Hypoport, Puma, Qiagen, Redcare Pharmacy | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| BIOGEN | 159,20 | -1,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,830 | -5,35 % | HEIDELBERG PHARMA AG unter Beobachtung | ||
| ILLUMINA | 111,08 | -2,30 % | Evercore ISI reiterates Illumina stock rating on competitive positioning | ||
| CRISPR THERAPEUTICS | 49,200 | -5,38 % | Is CRISPR Therapeutics Stock Too Risky to Buy Right Now? | ||
| INTELLIA THERAPEUTICS | 12,555 | -5,07 % | Intellia Therapeutics, Inc.: Intellia Therapeutics Announces FDA Lift of Clinical Hold on MAGNITUDE Phase 3 Clinical Trial in ATTR-CM | CAMBRIDGE, Mass., March 02, 2026 (GLOBE NEWSWIRE) -- Intellia Therapeutics, Inc. (Nasdaq: NTLA), a leading biopharmaceutical company focused on revolutionizing medicine leveraging CRISPR gene editing... ► Artikel lesen | |
| NUREXONE BIOLOGIC | 0,425 | +2,66 % | Exosomen auf dem Prüfstand: NurExone legt den Grundstein für die erste klinische Studie am Menschen | ||
| GUBRA | 50,85 | -1,55 % | Gubra A/S: Annual Report 2025: A record year | Today, Gubra releases its Annual Report for 2025 showing the strongest financial results in Gubra's history. Group revenue and operating profit amounted to DKK 2.6 billion and DKK 2.2 billion, respectively.... ► Artikel lesen | |
| ARROWHEAD PHARMACEUTICALS | 54,84 | -0,40 % | S&P Dow Jones Indices: Ciena Set to Join S&P 500; Arrowhead Pharmaceuticals to Join S&P MidCap 400; ADT and OneSpaWorld Holdings to Join S&P SmallCap 600 | NEW YORK, Feb. 4, 2026 /PRNewswire/ -- S&P Dow Jones Indices will make the following changes to the S&P 500, S&P MidCap 400, S&P SmallCap 600:
S&P MidCap 400... ► Artikel lesen | |
| GALAPAGOS NV | 28,900 | 0,00 % | RBC Capital raises Galapagos stock price target to $33 on model updates | ||
| SOL GLOBAL INVESTMENTS | 0,056 | -4,31 % | SOL Global Investments Corp (2): SOL Global president Marcotti resigns |
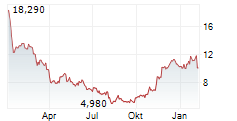
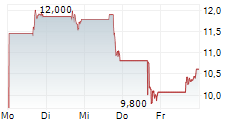
ENTRADA THERAPEUTICS INC gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 11,680 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ENTRADA THERAPEUTICS INC finden Sie auf Wallstreet Online.