- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 23.02. | Fortress Biotech, Inc. - 8-K, Current Report | 5 | SEC Filings | ||
| 23.02. | Fortress Biotech, Inc.: Fortress Biotech's Subsidiary Cyprium Therapeutics Enters into Agreement to Sell Rare Pediatric Disease Priority Review Voucher for $205 Million | 242 | GlobeNewswire (Europe) | MIAMI, Feb. 23, 2026 (GLOBE NEWSWIRE) -- Fortress Biotech, Inc. (Nasdaq: FBIO) ("Fortress") and its majority-owned subsidiary, Cyprium Therapeutics, Inc. ("Cyprium"), today announced that Cyprium... ► Artikel lesen | |
| 14.01. | Fortress Biotech receives FDA approval for Zycubo to treat Menkes disease | 13 | Pharmaceutical Technology | ||
| 13.01. | Fortress Bio gains on FDA approval of copper replacement therapy | 6 | Seeking Alpha | ||
| FORTRESS BIOTECH Aktie jetzt für 0€ handeln | |||||
| 13.01. | Fortress Biotech, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 13.01. | Fortress Biotech, Inc.: Fortress Biotech and Cyprium Therapeutics Announce U.S. FDA Approval of ZYCUBO (copper histidinate), the First and Only Approved Treatment for Menkes Disease in the United States | 787 | GlobeNewswire (Europe) | Rare Pediatric Disease Priority Review Voucher (PRV) granted by FDA at approval to be transferred from Sentynl Therapeutics to Cyprium Cyprium eligible to receive tiered royalties and up to $129 million... ► Artikel lesen | |
| 15.12.25 | FDA sets new PDUFA date for Fortress Biotech's Menkes disease therapy | 31 | Investing.com | ||
| 15.12.25 | Fortress Biotech, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 14.11.25 | Fortress Biotech, Inc.: Fortress Biotech Reports Third Quarter 2025 Financial Results and Recent Corporate Highlights | 651 | GlobeNewswire (Europe) | Total net revenue increased 20.5% to $17.6 million for third quarter of 2025 compared to the third quarter of 2024 Fortress subsidiary Checkpoint Therapeutics acquired by Sun Pharma; Fortress received... ► Artikel lesen | |
| 14.11.25 | Fortress Biotech, Inc. - 10-Q, Quarterly Report | 3 | SEC Filings | ||
| 14.11.25 | Fortress Biotech, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 11.11.25 | Fortress Biotech Q3 Earnings Preview | 5 | Seeking Alpha | ||
| 21.10.25 | Fortress Biotech: Aktie legt zu - Gichtmittel der Tochtergesellschaft erreicht Phase-3-Studie | 16 | Investing.com Deutsch | ||
| 21.10.25 | Fortress Biotech stock rises after subsidiary's gout treatment enters Phase 3 trials | 3 | Investing.com | ||
| 21.10.25 | Fortress Biotech, Inc.: Fortress Biotech and Subsidiary Urica Therapeutics Announce First Patients Dosed in Crystalys Therapeutics' Global Phase 3 Trials of Dotinurad for the Treatment of Gout | 281 | GlobeNewswire (Europe) | MIAMI, Oct. 21, 2025 (GLOBE NEWSWIRE) -- Urica Therapeutics, Inc. ("Urica" or the "Company"), a Fortress Biotech, Inc. (Nasdaq: FBIO) ("Fortress") subsidiary, today announced that Crystalys Therapeutics... ► Artikel lesen | |
| 02.10.25 | FDA verweigert Zulassung für Medikament von Fortress Biotech wegen Produktionsmängeln | 20 | Investing.com Deutsch | ||
| 02.10.25 | Fortress Biotech stock faces setback as FDA issues CRL for CUTX-101 | 3 | Investing.com | ||
| 01.10.25 | Fortress Biotech stock plummets after FDA rejects Menkes disease drug | 13 | Investing.com | ||
| 01.10.25 | Fortress Biotech plunges as FDA rejects rare disease drug | 20 | Seeking Alpha | ||
| 01.10.25 | Fortress Biotech, Inc. - 8-K, Current Report | 5 | SEC Filings |
1 2 Weiter >>
27 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 87,05 | -0,51 % | BioNTech-Konkurrent Moderna: Satter Kurssprung - jetzt noch einsteigen? | Die Aktie von Moderna hat zuletzt erneut kräftigen Rückenwind erhalten. Die Korrektur seit Ende Januar wurde damit nicht nur vollständig wieder ausgeglichen, das Papier konnte sogar auf ein neues Jahreshoch... ► Artikel lesen | |
| EVOTEC | 5,402 | -1,13 % | Shortseller-Positionen aktuell: Evotec, Gerresheimer, HelloFresh, Renk, Hypoport, Symrise, TUI | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| BB BIOTECH | 49,800 | -0,60 % | Christian Koch, Head BB Biotech: «Opportunitäten entstehen dort, wo Fortschritt und Bewertung noch nicht im Gleichgewicht sind» | ||
| MEDIGENE | 0,034 | -11,86 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 39,095 | -3,59 % | Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 45,130 | -0,29 % | Moderna freut sich über eine Zulassungsempfehlung der EMA für einen Kombi-Impfstoff gegen Corona und Grippe | Bei Corona-Impfstoffen war der US-Konzern Moderna noch ein Nachzügler. Anders scheint es nun bei Kombi-Impfstoffen zu laufen, die gleichzeitig vor Corona und der Grippe schützen. Dafür gibt es bisher... ► Artikel lesen | |
| PAION | 0,061 | -21,54 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,548 | -0,48 % | Valneva-Aktie: Die letzte Chance | Für den französischen Impfstoffentwickler Valneva entscheidet sich 2026 ein großer Teil der Investmentstory. Die Aktie wird derzeit fast ausschließlich durch die Erwartung der Phase-3-Daten zum Lyme-Borreliose-Impfstoff... ► Artikel lesen | |
| AMGEN | 316,00 | -0,74 % | Leerink Partners senkt Amgen-Prognose für Q1 wegen Lagerbestandsabbau | ||
| EPIGENOMICS | 0,960 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,567 | -0,22 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 314,60 | +0,16 % | William Blair reiterates Outperform on Stryker stock after analyst briefing | ||
| BIOGEN | 157,65 | -1,00 % | Biogen Inc.: The New England Journal of Medicine Publishes First Data to Demonstrate the Potential for Disease Modification in Dravet Syndrome | -Results show that targeting the underlying cause of Dravet syndrome with zorevunersen may improve outcomes for people with this rare, devastating genetic neurodevelopmental disease-
-Data support... ► Artikel lesen | |
| BIOFRONTERA | 2,680 | +2,68 % | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 3,030 | -2,26 % | EQS-Adhoc: Heidelberg Pharma AG: Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von Soleus Capital bekannt | EQS-Ad-hoc: Heidelberg Pharma AG / Schlagwort(e): Bedeutende Verträge
Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von... ► Artikel lesen |
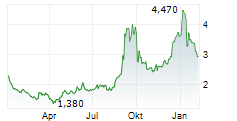
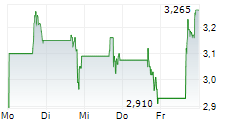
FORTRESS BIOTECH INC gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell +7,05 % bei einem Aktienkurs von 3,340 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu FORTRESS BIOTECH INC finden Sie auf Wallstreet Online.