- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| IPSEN SA ADR Aktie jetzt für 0€ handeln | |||||
| Mo | CDMO Quotient extends Ipsen supply pact for rare disease drug Sohonos | 1 | FiercePharma | ||
| Mo | CHMP backs Ipsen drug for childhood brain cancer | - | pharmaphorum | ||
| Fr | Ipsen Pharma: Ipsen receives positive CHMP opinion for Ojemda for the treatment as monotherapy of children with relapsed or refractory BRAF-altered pediatric low-grade glioma | 396 | GlobeNewswire (Europe) | If approved, Ojemda (tovorafenib) is expected to be the first and only targeted medicine in European Union for children with relapsed or refractory BRAF-altered pediatric low-grade glioma, irrespective... ► Artikel lesen | |
| Fr | Quotient and Ipsen extend partnership for ultra-rare disease therapy | 2 | Pharmaceutical Technology | ||
| 19.02. | Ipsen Pharma: Ipsen S.A. publishes its 2025 Consolidated Financial Statements | 1 | GlobeNewswire (USA) | ||
| 12.02. | Genfit adds $20M as Ipsen records strong sales for liver drug | 6 | Seeking Alpha | ||
| 12.02. | GENFIT S.A.: GENFIT to receive US$20M milestone after Ipsen's Iqirvo exceeds the US$200M threshold in its first full year of net sales | 231 | GlobeNewswire (Europe) | Ipsen reported US$88M of Iqirvo® net sales in PBC for 4Q25, bringing 2025 sales to US$208M, triggering the first commercial milestone payment to GENFIT ahead of schedule1Ipsen confirmed its commitment... ► Artikel lesen | |
| 12.02. | Ipsen GJ 2025: Zweistelliges Wachstum beflügelt ambitionierten Ausblick für 2026 | 2 | Investing.com Deutsch | ||
| 12.02. | Ipsen übertrifft Prognosen in Q4 2025 - Aktie legt deutlich zu | 6 | Investing.com Deutsch | ||
| 12.02. | Ipsen Full-Year Profit Rises On Higher Sales; Sees Over 13% Sales Growth In 2026 | 364 | AFX News | PARIS (dpa-AFX) - Ipsen S.A. (IPSEY), a specialty-care biopharmaceutical company, on Thursday reported higher earnings for 2025, supported by stronger sales performance.Net income attributable... ► Artikel lesen | |
| 12.02. | Ipsen S.A. Non-GAAP EPS of €12.09, revenue of €3.68B; sets full-year 2026 guidance and mid-term outlook | 6 | Seeking Alpha | ||
| 12.02. | Ipsen Pharma: Ipsen delivers strong results in 2025, driven by solid execution across all therapeutic areas, and provides 2026 guidance | 651 | GlobeNewswire (Europe) | FY 2025 sales growth of 10.9% at CER1, or 8.1% as reported, driven by growth of the three therapeutic areas of Oncology (4.1%1), Rare Disease (102.5%1) and Neuroscience (9.7%1); Somatuline sales growth... ► Artikel lesen | |
| 09.02. | Ipsen Pharma: Ipsen - January 2026 - Monthly information relative to the total number of voting rights and shares composing the share capital | 1 | GlobeNewswire (USA) | ||
| 29.01. | Ipsen Pharma: Ipsen nominates Peter Guenter to its Board of Directors | 449 | GlobeNewswire (Europe) | PARIS, FRANCE, 29 January 2026 - Ipsen (Euronext: IPN; ADR: IPSEY) announced today the co-optation of Peter Guenter to its Board as a Director, effective January 28, 2026, further to the vacancy of... ► Artikel lesen | |
| 28.01. | Ipsen appoints Pierrick Lefranc as Executive Vice President Technical Operations | 3 | PMLiVE | ||
| 27.01. | Ipsen Pharma: Pierrick Lefranc appointed as Executive Vice President Technical Operations, member of Executive Leadership Team | 460 | GlobeNewswire (Europe) | PARIS, FRANCE, 27 January 2026 - Ipsen (Euronext: IPN; ADR: IPSEY) announced today that Pierrick Lefranc will be appointed as Executive Vice President (EVP) and member of the Executive Leadership Team... ► Artikel lesen | |
| 22.01. | Galderma und Ipsen beenden F&E-Partnerschaft für Neuromodulatoren nach Schiedsspruch | 20 | Investing.com Deutsch | ||
| 21.01. | Ipsen Pharma: Arbitration tribunal upholds Ipsen's termination of R&D agreement with Galderma | 481 | GlobeNewswire (Europe) | PARIS, FRANCE, 21 January 2026 - Ipsen (Euronext: IPN; ADR: IPSEY) announced that the Arbitral Tribunal of the International Chamber of Commerce (ICC) has issued a final decision in favor of Ipsen,... ► Artikel lesen | |
| 14.01. | Ipsen rises on FDA breakthrough therapy status for blood cancer therapy | 3 | Seeking Alpha | ||
| 14.01. | Ipsen Pharma: New data reinforces Ipsen's commitment to bringing solutions and addressing care gaps in neurological diseases at TOXINS | 793 | GlobeNewswire (Europe) | 14 abstracts will be presented across a range of neurological conditions including post-stroke spasticity, cervical dystonia, blepharospasm and other movement disordersInterim data from the ongoing... ► Artikel lesen |
1 2 3 4 5 Weiter >>
104 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 38,645 | -4,84 % | MustGrow Biologics und die Partnerschaft mit Bayer: Darum steht die Aktie erst am Anfang | Die globale Landwirtschaft steht vor einem Paradox. Sie muss mehr Menschen ernähren, aber mit weniger chemischen Mitteln. Weltweit sind in 162 Ländern inzwischen 460 Pestizide verboten oder eingeschränkt.... ► Artikel lesen | |
| NOVO NORDISK | 31,320 | -2,64 % | maydornsmeinung: Silber, Nvidia, AMD, Microsoft, IBM, SAP, TeamViewer, Novo Nordisk, Nebius & Co. | In maydornsmeinung geht es zunächst um den Gesamtmarkt: Trotz Zoll-Chaos bleiben für Alfred die Unternehmensgewinne der entscheidende Faktor. Die Berichtssaison läuft stark, auch wenn sich die großen... ► Artikel lesen | |
| MERCK KGAA | 122,00 | -3,37 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| ATRIUM THERAPEUTICS | 15,060 | 0,00 % | Atrium Therapeutics Launches with Approximately $270 Million to Advance Novel RNA Medicines for Rare Genetic Cardiomyopathies | Spinoff from Novartis AG's acquisition of Avidity Biosciences advances precision cardiology programs using targeted RNA delivery platform
Lead candidates ATR 1072... ► Artikel lesen | |
| HARROW | 53,29 | 0,00 % | Why Is Eye-Disease-Focused Harrow Stock Falling Today? | ||
| PFIZER | 23,175 | -0,56 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| GSK | 24,610 | -1,28 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| SANOFI | 80,76 | -0,93 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| AQUESTIVE THERAPEUTICS | 4,225 | 0,00 % | Aquestive Therapeutics, Inc. - 8-K, Current Report | ||
| GENERATE BIOMEDICINES | 12,420 | 0,00 % | Generate Biomedicines Prices IPO At $16/shr | WASHINGTON (dpa-AFX) - Generate Biomedicines, Inc. (GENB) on Thursday priced its initial public offering of 25,000,000 shares of stock at $16 per share, raising gross proceeds of $400 million.The... ► Artikel lesen | |
| ELI LILLY | 869,70 | -0,09 % | Aktien New York Ausblick: Stabile Kurse vor Rede von Trump - AMD-Aktie stark | NEW YORK (dpa-AFX) - Nach dem sehr schwachen Wochenauftakt stehen die Vorzeichen für die US-Börsen am Dienstag auf Stabilisierung. Größere Sprünge nach oben sind aber nicht zu erwarten. Stattdessen... ► Artikel lesen | |
| NOVARTIS | 140,28 | -1,93 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| DERMAPHARM | 37,850 | -4,54 % | Dermapharm: Spannend für langfristig orientierte Investoren | Am 17. März wird Dermapharm Zahlen für 2025 präsentieren. Erwartet wird von Dermapharm ein Umsatz von 1,16 Milliarden Euro bis 1,2 Milliarden Euro. Das bereinigte EBITDA sieht die Gesellschaft bei 322... ► Artikel lesen | |
| MERCK & CO | 103,20 | -0,58 % | Tempus AI expands collaboration with Merck on precision medicine | ||
| LB PHARMACEUTICALS | 23,900 | 0,00 % | LB Pharmaceuticals Inc: LB Pharmaceuticals Announces $100.0 Million Private Placement | NEW YORK, Feb. 05, 2026 (GLOBE NEWSWIRE) -- LB Pharmaceuticals Inc ("LB Pharmaceuticals" or the "Company") (Nasdaq: LBRX), a late-stage biopharmaceutical company developing novel therapies for schizophrenia... ► Artikel lesen |
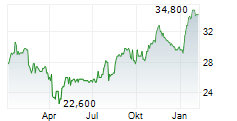
IPSEN SA ADR gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 41,200 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu IPSEN SA ADR finden Sie auf Wallstreet Online.