- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Aquestive Therapeutics Q4 2025 Earnings Preview | 2 | Seeking Alpha | ||
| Do | Aquestive Therapeutics, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 23.02. | Lake Street Adjusts Aquestive Therapeutics, Inc. (AQST) Valuation Model on Expected Approval Delay | 3 | Insider Monkey | ||
| 20.02. | Aquestive Therapeutics, Inc.: Aquestive Therapeutics to Present New Clinical Data on Anaphylm (dibutepinephrine) Sublingual Film at the 2026 AAAAI Annual Meeting | 7 | GlobeNewswire (USA) | ||
| 18.02. | Aquestive Therapeutics Names Matthew Greenhawt As Chief Medical Officer | 1 | RTTNews | ||
| AQUESTIVE THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 18.02. | Aquestive Therapeutics, Inc.: Aquestive Therapeutics Appoints Internationally Recognized Allergist Dr. Matthew Greenhawt as Chief Medical Officer | 789 | GlobeNewswire (Europe) | Board-certified allergist and leading food allergy and anaphylaxis researcher joins Aquestive to support Anaphylm (dibutepinephrine) sublingual film NDA resubmission and pipeline WARREN, N.J., Feb.... ► Artikel lesen | |
| 06.02. | Weekly Buzz: ATYR To Meet With FDA, SGMO Aces Fabry Study, AQST's Anaphylm Gets Rejected | 663 | AFX News | PETAH TIKVA (dpa-AFX) - This week's biotech landscape witnessed key FDA meetings being scheduled, IND clearances, complete response letters, sale of oncology assets and clinical trial data readouts... ► Artikel lesen | |
| 03.02. | H.C. Wainwright reiterates Buy rating on Aquestive stock after FDA response | 6 | Investing.com | ||
| 03.02. | Aquestive receives FDA CRL for Anaphylm allergic reaction treatment | 3 | Pharmaceutical Technology | ||
| 02.02. | Aquestive jumps despite receiving FDA Complete Response Letter | 9 | Seeking Alpha | ||
| 02.02. | What's Going On With Aquestive Therapeutics Stock After FDA Update For Lead Allergic Treatment? | 1 | Benzinga.com | ||
| 02.02. | With FDA rejections, Aquestive's shares go up, while Pharming's go down | 16 | FiercePharma | ||
| 02.02. | Aquestive Receives CRL For Anaphylm NDA; Deficiencies Limited To Packaging, Administration | 2 | RTTNews | ||
| 02.02. | Aquestive-Aktie steigt trotz vorläufiger FDA-Ablehnung für Anaphylaxie-Film | 7 | Investing.com Deutsch | ||
| 02.02. | FDA issues complete response letter for Aquestive's anaphylaxis drug | 1 | Investing.com | ||
| 02.02. | Aquestive Therapeutics, Inc.: Aquestive Therapeutics Announces FDA Issuance of Complete Response Letter for Anaphylm | 1.092 | GlobeNewswire (Europe) | Deficiencies limited to packaging and administrationCompany believes it can rapidly resolve deficiencies and expects to resubmit as early as Q3 2026Remains well-capitalized and anticipates ending... ► Artikel lesen | |
| 02.02. | Aquestive Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 21.01. | Aquestive Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 6 | SEC Filings | ||
| 13.01. | Block & Leviton LLP: Aquestive Therapeutics Investors Who Have Lost Money Should Contact Block & Leviton to Find Out How They Might Recover Money Through the Firm's Investigation | 695 | Newsfile | Boston, Massachusetts--(Newsfile Corp. - January 13, 2026) - Block & Leviton is investigating Aquestive Therapeutics, Inc. (NASDAQ: AQST) for potential securities law violations. Investors who have... ► Artikel lesen | |
| 10.01. | Benzinga Bulls And Bears: Chevron, Palantir, Aquestive - And Real Estate Stocks Plummet | 29 | Benzinga.com |
1 2 3 Weiter >>
45 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 37,255 | -2,75 % | Bayer verklagt Johnson & Johnson - Aktie fällt und fällt | Der DAX-Konzern Bayer will sich in den Vereinigten Staaten juristisch gegen Johnson & Johnson wehren. Der Hintergrund für die Klage ist potenziell irreführende Werbung für ein Prostatakrebs-Medikament... ► Artikel lesen | |
| NOVO NORDISK | 32,050 | +1,78 % | Novo Nordisk: Immer tiefer - wie weit kann die Aktie noch fallen? | Die Aktie von Novo Nordisk ist am Montag dieser Woche erneut massiv unter Druck geraten. Grund waren enttäuschende Studienergebnisse beim Hoffnungsträger CagriSema. Analysten senkten daraufhin ihre... ► Artikel lesen | |
| PFIZER | 22,890 | +0,02 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 140,42 | -0,45 % | Novartis baut fünften Standort für Radioliganden-Therapie in den USA | DJ Novartis baut fünften Standort für Radioliganden-Therapie in den USA
DOW JONES--Der Schweizer Pharmakonzern Novartis hat den Bau eines weiteren Standortes für Radioligandentherapie (RLT) in... ► Artikel lesen | |
| MERCK KGAA | 122,65 | +1,07 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 127,48 | +0,17 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,020 | +0,33 % | Canaccord Initiates Aurora Cannabis Inc. (ACB) With C$10 Price Target | ||
| SANOFI | 80,30 | -0,21 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,600 | +1,03 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| ABBVIE | 202,00 | +0,25 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 397,55 | +0,20 % | Erfolg für ROCHE | Der Schweizer Pharmakonzern hat mit seinem Medikamentenkandidat gegen Multiple Sklerose das primäre Ziel einer klinischen Studie in der Spätphase bei der häufigsten Form der Erkrankung erreicht. Die... ► Artikel lesen | |
| CANOPY GROWTH | 0,909 | -0,87 % | Is Canopy Growth Stock Going to $0? | ||
| ELI LILLY | 866,10 | -0,12 % | Eli Lilly und Novo Nordisk: Angst vor einem Preiskrieg bringt Aktien unter Druck | Novo Nordisk will ab 2027 die Preise für seine Blockbuster-Medikamente Wegovy und Ozempic in den USA drastisch senken und setzt damit Wettbewerber Eli Lilly unter Zugzwang. Die Aktien der beiden Pharmakonzerne... ► Artikel lesen | |
| ASTRAZENECA | 173,55 | -0,09 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TILRAY BRANDS | 6,390 | +0,95 % | MDMA-Herausforderer Bioxyne punktet mit 112% Umsatzplus. Und was macht Tilray? |
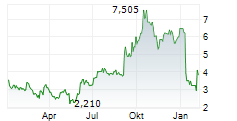
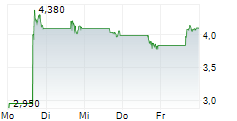
AQUESTIVE THERAPEUTICS INC gehört der Branche Pharma an. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 4,225 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu AQUESTIVE THERAPEUTICS INC finden Sie auf Wallstreet Online.