- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 05.02. | Lexeo Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 27.01. | Lexeo Therapeutics stellt Führungsteam neu auf und ernennt Chief Medical Officer | - | Investing.com Deutsch | ||
| 27.01. | Lexeo Therapeutics announces key leadership appointments | 1 | Investing.com | ||
| 27.01. | Lexeo Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 13.01. | Oppenheimer reiterates Outperform rating on Lexeo Therapeutics stock | 5 | Investing.com | ||
| 13.01. | Lexeo Therapeutics stock falls 23% despite Cantor maintaining Overweight rating | 1 | Investing.com | ||
| LEXEO THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 13.01. | H.C. Wainwright lowers Lexeo Therapeutics stock price target on PKP2 data concerns | 1 | Investing.com | ||
| 12.01. | Aktie von Lexeo Therapeutics bricht nach Studiendaten zur Gentherapie ein | 5 | Investing.com Deutsch | ||
| 12.01. | Lexeo Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 12.01. | Lexeo: Positive Studiendaten zu Gentherapie bei Herzerkrankung | 3 | Investing.com Deutsch | ||
| 12.01. | Lexeo's gene therapy shows promising results for heart condition | 3 | Investing.com | ||
| 12.01. | Lexeo Therapeutics Announces Positive Interim Phase I/II Data for LX2020 for the Treatment of PKP2-Associated Arrhythmogenic Cardiomyopathy | 134 | GlobeNewswire (Europe) | LX2020 generally well tolerated across ten participants with no clinically significant complement activation LX2020 transduction, transcription, and increased protein expression observed across participants... ► Artikel lesen | |
| 08.01. | Lexeo Therapeutics Partners With JNJ To Investigate Localized Cardiac Delivery Of Gene Therapy | 386 | AFX News | NEW BRUNSWICK (dpa-AFX) - Lexeo Therapeutics, Inc. (LXEO), Thursday announced a research collaboration with Johnson & Johnson (JNJ) to investigate localized cardiac delivery of gene therapy.Under... ► Artikel lesen | |
| 08.01. | Lexeo Therapeutics Announces Research Collaboration to Explore Targeted Cardiac Delivery of AAV Gene Therapy | 160 | GlobeNewswire (Europe) | Collaboration will combine Lexeo expertise in cardiac genetic medicine with Johnson & Johnson's expertise in cardiovascular therapeutics and circulatory technologies, including Impella heart pumps... ► Artikel lesen | |
| 27.12.25 | Wall Street Rallies Behind Lexeo Therapeutics (LXEO)'s Gene Therapy Pipeline | 3 | Insider Monkey | ||
| 17.12.25 | Raymond James initiates Lexeo Therapeutics stock with Strong Buy rating | 3 | Investing.com | ||
| 10.12.25 | Lexeo Therapeutics stock holds Overweight rating as Cantor sees PKP2-ACM potential | 1 | Investing.com | ||
| 10.12.25 | Oppenheimer reiterates Outperform rating on Lexeo Therapeutics stock | 1 | Investing.com | ||
| 01.12.25 | Lexeo Therapeutics stock undervalued, Cantor Fitzgerald reiterates $19 target | 2 | Investing.com | ||
| 01.12.25 | Cantor Fitzgerald stuft Lexeo Therapeutics als unterbewertet ein und bestätigt Kursziel von 19 $ | 1 | Investing.com Deutsch |
1 2 3 Weiter >>
47 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| SANGAMO THERAPEUTICS | 0,372 | +0,79 % | Weekly Buzz: ATYR To Meet With FDA, SGMO Aces Fabry Study, AQST's Anaphylm Gets Rejected | PETAH TIKVA (dpa-AFX) - This week's biotech landscape witnessed key FDA meetings being scheduled, IND clearances, complete response letters, sale of oncology assets and clinical trial data readouts... ► Artikel lesen | |
| AVID BIOSERVICES | - | - | Avid Bioservices Announces Grand Opening of New Early Phase Center of Excellence in Costa Mesa, California | COSTA MESA, Calif., Feb. 24, 2026 /PRNewswire/ -- Avid Bioservices, Inc., a dedicated biologics CDMO, today announced the grand opening of its new Early Phase Center of Excellence, a state... ► Artikel lesen | |
| PROTALIX BIOTHERAPEUTICS | 2,480 | +0,81 % | Chiesi Global Rare Diseases and Protalix BioTherapeutics Receive Positive CHMP Opinion for an Additional Dosing Regimen of Every Four Weeks for Elfabrio (pegunigalsidase alfa) in the EU | Committee
for
Medicinal
Products
for
Human
Use
(CHMP)
issues
a
positive
opinion following re-examination, which
will
be reviewed by
the... ► Artikel lesen | |
| REPLIMUNE | 6,450 | -0,77 % | Replimune, Inc.: Replimune Reports Fiscal Third Quarter 2026 Financial Results and Provides Corporate Update | WOBURN, Mass., Feb. 03, 2026 (GLOBE NEWSWIRE) -- Replimune Group, Inc. (Nasdaq: REPL), a clinical stage biotechnology company pioneering the development of novel oncolytic immunotherapies, today announced... ► Artikel lesen | |
| QUANTERIX | 6,550 | -2,46 % | Quanterix Corporation: Quanterix Announces Landmark Nature Study Redefining Alzheimer's Disease Neuropathology Prevalence | Simoa p-Tau 217 Assay Enables Largest Population-Based Assessment (11,000+ Samples) Revealing Striking Age-Related Increase and Broad Treatment Eligibility
Quanterix Corporation(NASDAQ: QTRX),... ► Artikel lesen | |
| BELITE BIO | 160,00 | +1,27 % | Exploring Belite Bio's Earnings Expectations | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BIONTECH | 93,10 | -0,21 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | -79,74 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | +24,33 % | Cardio Diagnostics Holdings, Inc. - 8-K, Current Report |
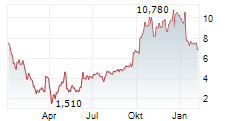
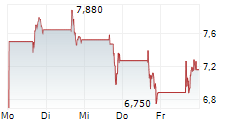
LEXEO THERAPEUTICS INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von +0,310 (+4,50 %) liegt der Kurs bei 7,200 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu LEXEO THERAPEUTICS INC finden Sie auf Wallstreet Online.