| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
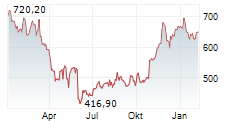
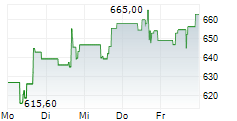
| 655,80 | 657,00 | 22:09 | |
| 653,40 | 658,40 | 22:00 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Regeneron to present Phase 3 data on novel allergy antibodies at AAAAI | 2 | Investing.com | ||
| Di | Regeneron Pharmaceuticals, Inc.: Regeneron Highlights Expanding Immunology Portfolio and Pipeline at AAAAI, Showcasing Novel Approaches to Treating Allergy | 1.664 | GlobeNewswire (Europe) | 36 abstracts to be presented across Regeneron-invented therapies, including first-time Phase 3 presentations for two distinct investigational allergen-blocking antibodies for cat and birch allergies... ► Artikel lesen | |
| 04.02. | REGENERON PHARMACEUTICALS, INC. - 10-K, Annual Report | 20 | SEC Filings | ||
| 02.02. | These Analysts Revise Their Forecasts On Regeneron Pharmaceuticals After Q4 Results | 8 | Benzinga.com | ||
| 02.02. | BMO Capital bestätigt "Outperform"-Rating für Regeneron-Aktie mit Kursziel 850 $ | 8 | Investing.com Deutsch | ||
| REGENERON PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| 02.02. | BMO Capital reiterates Outperform rating on Regeneron stock at $850 | 6 | Investing.com | ||
| 02.02. | Cantor Fitzgerald hebt Kursziel für Regeneron wegen Portfolio-Aussichten auf 800 US-Dollar an | 6 | Investing.com Deutsch | ||
| 02.02. | Regeneron Pharmaceuticals raises dividend by 6.8% to $0.94 | 6 | Seeking Alpha | ||
| 02.02. | Cantor Fitzgerald raises Regeneron stock price target to $800 on portfolio outlook | 4 | Investing.com | ||
| 02.02. | Bernstein raises Regeneron Pharma stock price target to $925 on solid quarter | 4 | Investing.com | ||
| 02.02. | Regeneron to present new EYLEA HD data at Angiogenesis meeting | 2 | Investing.com | ||
| 02.02. | Regeneron Pharmaceuticals, Inc.: EYLEA HD (aflibercept) Injection 8 mg Presentations at Angiogenesis 2026 Underscore Strength of its Clinical Profile for the Treatment of Serious Retinal Diseases | 5 | GlobeNewswire (USA) | ||
| 02.02. | Wells Fargo raises Regeneron stock price target to $800 on Dupixent outlook | 2 | Investing.com | ||
| 02.02. | Dupixent-Ausblick: Wells Fargo hebt Regeneron-Kursziel auf 800 Dollar an | 3 | Investing.com Deutsch | ||
| 31.01. | Regeneron (REGN) Draws Higher Target From TD Cowen | 2 | Insider Monkey | ||
| 30.01. | Regeneron outlines 18 new Phase III studies and expects four FDA approvals in 2026 while advancing EYLEA HD and DUPIXENT growth | 4 | Seeking Alpha | ||
| 30.01. | Baird raises Regeneron Pharma stock price target to $742 on earnings beat | 7 | Investing.com | ||
| 30.01. | Regeneron: Baird hebt Kursziel nach starken Quartalszahlen auf 742 US-Dollar an | 16 | Investing.com Deutsch | ||
| 30.01. | A sales turnaround for Regeneron's Eylea franchise will have to wait at least another quarter | 3 | FiercePharma | ||
| 30.01. | Regeneron Q4 2025 slides: Dupixent growth drives revenue, EYLEA HD gains momentum | 21 | Investing.com |
1 2 3 4 5 Weiter >>
336 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 92,35 | +0,82 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 5,992 | -2,92 % | SCHOCKMELDUNG bei Evotec: Aktie bricht ein - droht jetzt der Absturz? | ||
| BB BIOTECH | 49,700 | -1,19 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,040 | -7,80 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,000 | -0,43 % | Qiagen übertrifft Markterwartungen im vierten Quartal | DJ Qiagen übertrifft Markterwartungen im vierten Quartal
DOW JONES--Qiagen hat im Schlussquartal 2025 den Umsatz gesteigert, der operative Gewinn ging gegenüber dem Vorjahr zurück. 2026 rechnet... ► Artikel lesen | |
| MODERNA | 34,060 | -3,53 % | Moderna-Aktie -9%: Trotz der Hiobsbotschaft kaufen? | Nachdem die Moderna-Aktie bereits in den letzten zwei Handelswochen den Rückwärtsgang eingeschaltet hat, bricht sie am Mittwochmorgen um -9% im europäischen Handel ein. Was für eine Hiobsbotschaft steckt... ► Artikel lesen | |
| PAION | 0,160 | -11,11 % | PAION AG zeigt strategische Stärke | ||
| VALNEVA | 4,084 | -1,50 % | VALNEVA Declaration of shares and voting rights: January 31, 2026 | ||
| AMGEN | 308,05 | +0,51 % | Märkte am Morgen: "KI-Realitätscheck", Wolters Kluwer, AMD, Amgen, Walmart, Novo Nordisk | Die Stimmung an den Aktienmärkten ist derzeit sehr, sehr wechselhaft. Das war gestern deutlich zu spüren. Der DAX war bemerkenswert stark in den Handel gestartet, schloss allerdings leicht im Minus.... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 7,762 | +5,45 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 306,70 | +1,02 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 161,10 | -0,43 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,400 | +0,84 % | Biofrontera's Ameluz SNDA For Superficial Basal Cell Carcinoma Accepted By FDA | ||
| HEIDELBERG PHARMA | 3,100 | +6,53 % | Heidelberg Pharma erhält Meilensteinzahlung von Takeda | Die Heidelberg Pharma AG hat im Rahmen einer bestehenden Lizenzvereinbarung eine Meilensteinzahlung von Takeda erhalten. Die Zahlung wurde mit der Dosierung des ersten Patienten in einer klinischen... ► Artikel lesen |
Das Unternehmen REGENERON PHARMACEUTICALS INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +3,21 % bei einem Aktienkurs von 655,00 Euro. Aus der Ausschüttung der letzten 12 Monate von insgesamt 3,01 Euro errechnet sich eine Dividendendrendite von +0,46 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu REGENERON PHARMACEUTICALS INC finden Sie auf Wallstreet Online.