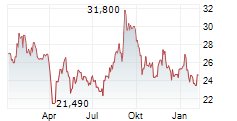
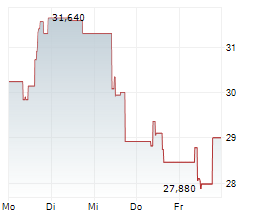
TOKYO (dpa-AFX) - BioArctic AB (BRCTF) announced on Monday that its partner Eisai (ESALY) has received Priority Review from the U.S. Food and Drug Administration (FDA) for the supplemental Biologics License Application (sBLA) for the Leqembi Iqlik subcutaneous autoinjector.
The Prescription Drug User Fee Act (PDUFA) action date is set for May 24, 2026.
Leqembi is currently approved in more than 50 countries and regions for the treatment of early Alzheimer's disease.
If approved, the Leqembi Iqlik subcutaneous (SC) autoinjector could provide an alternative to the current intravenous (IV) dosing method.
The sBLA is supported by positive data from the Phase 3 Clarity Alzheimer's disease open-label extension (OLE) study, which evaluated SC administration of lecanemab across various doses.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News