- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 05:59 | Aardvark Therapeutics Pauses Phase 3 Trial Of ARD-101 In Prader-Willi Syndrome; Stock Plunges | 10 | RTTNews | ||
| Fr | Aardvark Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| Fr | Aardvark Therapeutics, Inc.: Aardvark Therapeutics Announces Voluntary Pause of Phase 3 HERO Trial in Prader-Willi Syndrome | 49 | GlobeNewswire (Europe) | SAN DIEGO, Feb. 27, 2026 (GLOBE NEWSWIRE) -- Aardvark Therapeutics, Inc. (Aardvark) (Nasdaq: AARD), a clinical-stage biopharmaceutical company focused on developing novel, small-molecule therapeutics... ► Artikel lesen | |
| 13.02. | Aardvark Therapeutics, Inc.: Aardvark Therapeutics Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 2 | GlobeNewswire (USA) | ||
| 12.02. | Aardvark Therapeutics Establishes Ardia Subsidiary To Advance Dermatology Pipeline | 2 | RTTNews | ||
| 10.02. | Aardvark Therapeutics, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 28.01. | B.Riley initiates coverage on Aardvark Therapeutics stock with Buy rating | 4 | Investing.com | ||
| 28.01. | B.Riley nimmt Aardvark Therapeutics mit Kaufempfehlung in die Bewertung auf | 3 | Investing.com Deutsch | ||
| AARDVARK THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 12.01. | Aardvark Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 12.01. | Raymond James bestätigt "Strong Buy" für Aardvark Therapeutics mit Kursziel von 47 Dollar | 4 | Investing.com Deutsch | ||
| 12.01. | Raymond James reiterates Strong Buy on Aardvark Therapeutics stock with $47 target | 3 | Investing.com | ||
| 23.12.25 | Oppenheimer initiates Aardvark Therapeutics stock with Outperform rating | 2 | Investing.com | ||
| 23.12.25 | Oppenheimer startet Coverage für Aardvark Therapeutics mit "Outperform" | 3 | Investing.com Deutsch | ||
| 12.12.25 | William Blair initiates coverage on Aardvark Therapeutics stock with Outperform rating | 1 | Investing.com | ||
| 12.12.25 | William Blair nimmt Coverage für Aardvark Therapeutics mit "Outperform" auf | 4 | Investing.com Deutsch | ||
| 11.12.25 | Aardvark Therapeutics stock rises 13% on positive trial updates, BTIG maintains Buy | 1 | Investing.com | ||
| 11.12.25 | Raymond James bestätigt Aardvark Therapeutics mit "Strong Buy" und Kursziel von 47 $ | 3 | Investing.com Deutsch | ||
| 11.12.25 | Raymond James reiterates Strong Buy on Aardvark Therapeutics stock with $47 target | 4 | Investing.com | ||
| 10.12.25 | Aardvark Therapeutics, Inc.: Aardvark Therapeutics Announces First Patient Dosed in Australia in HERO Phase 3 Trial for Prader-Willi Syndrome | 208 | GlobeNewswire (Europe) | Multiple active sites and strong US enrollment continue to support Q3 2026 timing for topline data readout Clinical trial sites in Canada and the United Kingdom have received regulatory clearance... ► Artikel lesen | |
| 10.12.25 | Aardvark Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings |
1 2 3 Weiter >>
43 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| VIKING THERAPEUTICS | 28,340 | -1,19 % | Viking Therapeutics: The High-Stakes Weight Loss Contender | ||
| BIOCRYST PHARMACEUTICALS | 7,342 | -0,92 % | BioCryst Pharmaceuticals, Inc.: BioCryst Reports Full Year 2025 Financial Results and Provides Business Update | -Full year 2025 ORLADEYO® net revenue of $601.8 million (+38% y-o-y; +43% y-o-y excluding European ORLADEYO revenue following the sale of the European ORLADEYO business to Neopharmed Gentili S.p.A.... ► Artikel lesen | |
| IMMUNITYBIO | 8,228 | -0,75 % | ImmunityBio, Inc.: ImmunityBio Reports 700% Year-Over-Year Revenue Growth, Expanded ANKTIVA Approvals in Lung Cancer and Global Commercial Partnerships in 33 Countries with Label Expansion Plans Globally | 2025 Sales Momentum: ANKTIVA net product revenue increased 20% quarter-over-quarter, with full-year net product revenue of $113 million, representing an approximately 700% increase year-over-year... ► Artikel lesen | |
| MICROBOT MEDICAL | 2,038 | -0,29 % | H.C. Wainwright bestätigt Rating für Microbot Medical nach Adoption durch Klinik | ||
| CYTOKINETICS | 53,50 | +0,94 % | Cytokinetics, Incorporated: Cytokinetics Reports Fourth Quarter 2025 Financial Results and Provides Business Update | MYQORZO® Approved for Adults with Symptomatic Obstructive HCM in U.S., China and Europe; U.S. Launch Underway with First Prescriptions Dispensed within Days of Drug Availability Supplemental NDA for... ► Artikel lesen | |
| VOYAGER THERAPEUTICS | 3,426 | -1,44 % | Voyager Therapeutics, Inc.: Transition Bio and Voyager Announce Collaboration to Advance Small Molecules Targeting TDP-43 in ALS and Frontotemporal Dementia | CAMBRIDGE, United Kingdom and LEXINGTON, Mass., Nov. 10, 2025 (GLOBE NEWSWIRE) -- Transition Bio, a drug discovery company focused on unlocking traditionally "undruggable" targets by leveraging biomolecular... ► Artikel lesen | |
| CAPRICOR | 23,300 | -1,48 % | Capricor Therapeutics Provides Regulatory Update on Deramiocel BLA Following FDA Review of HOPE-3 Topline Data | FDA has requested the HOPE-3 clinical study report (CSR) as part of the BLA review processCompany expects to submit updates to the BLA in February 2026 to support continued FDA review SAN DIEGO,... ► Artikel lesen | |
| ARCTURUS THERAPEUTICS | 8,240 | -0,96 % | Arcturus Therapeutics Provides Interim Phase 2 Data for Cystic Fibrosis Program | ARCT-032 generally safe and well tolerated
Meaningful trends of clinical activity observed via high resolution CT scans
After only 28 days of treatment with ARCT-032 (10 mg), 4 out of 6 Class... ► Artikel lesen | |
| ABEONA THERAPEUTICS | 4,280 | -1,38 % | Abeona Therapeutics Inc.: Abeona Therapeutics Announces Appointment of Mohamad Tabrizi as Chief Business Officer | CLEVELAND, Dec. 15, 2025 (GLOBE NEWSWIRE) -- Abeona Therapeutics Inc. (Nasdaq: ABEO) today announced the appointment of Mohamad Tabrizi, M.S., M.B.A., as Senior Vice President, Chief Business Officer... ► Artikel lesen | |
| ZEVRA THERAPEUTICS | 7,500 | -1,32 % | Johnson Fistel, PLLP: Zevra Therapeutics Shareholders Are Encouraged to Reach Out to Johnson Fistel for More Information About Potentially Recovering Their Losses | San Diego, California--(Newsfile Corp. - February 19, 2026) - Johnson Fistel, PLLP is investigating potential claims on behalf of investors of Zevra Therapeutics, Inc. (NASDAQ: ZVRA). The investigation... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| PALVELLA THERAPEUTICS | 116,00 | +0,87 % | Palvella Therapeutics Inc.: Palvella Therapeutics Announces Positive Topline Results from Phase 3 SELVA Clinical Study of QTORIN 3.9% Rapamycin Anhydrous Gel (QTORIN rapamycin) in Microcystic Lymphatic Malformations | Primary endpoint met with statistically significant improvement (mean change of +2.13; p Achieved statistical significance on pre-specified key secondary endpoint (p 95% of trial participants aged... ► Artikel lesen | |
| KALVISTA PHARMACEUTICALS | 13,200 | -4,35 % | KalVista Pharmaceuticals, Inc.: KalVista Pharmaceuticals to Present at Upcoming Investor Conferences | KalVista Pharmaceuticals, Inc. (Nasdaq: KALV), today announced that the Company will participate in a fireside chat at each of the following upcoming investor conferences:
TD Cowen 46th Annual... ► Artikel lesen | |
| MARKER THERAPEUTICS | 1,190 | 0,00 % | Marker Therapeutics Reports Third Quarter 2025 Financial Results and Provides Business Updates | APOLLO study showed encouraging overall responses and favorable safety profile in relapsed/refractory B-cell lymphoma MT-601 demonstrated 66% objective response rate with 50% complete response in... ► Artikel lesen | |
| CARTESIAN THERAPEUTICS | 6,500 | +3,17 % | Cartesian Therapeutics, Inc.: Cartesian Therapeutics Highlights Recent Progress and Outlines 2026 Outlook | Enrollment on track in Phase 3 AURORA trial of Descartes-08 in myasthenia gravis IND application for Descartes-08 in myositis accepted by FDA; seamless adaptive clinical trial offering potential... ► Artikel lesen |
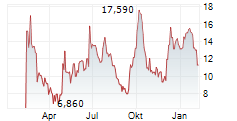
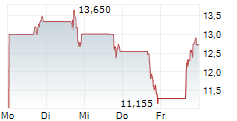
Das Unternehmen AARDVARK THERAPEUTICS INC kann der Branche Biotechnologie zugeordnet werden. Mit einer aktuellen Kursveränderung von +0,015 (+0,12 %) liegt der Kurs bei 12,495 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu AARDVARK THERAPEUTICS INC finden Sie auf Wallstreet Online.