| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
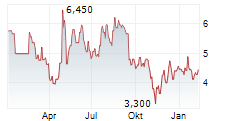
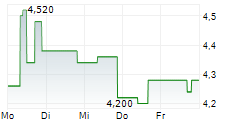
| 4,260 | 4,300 | 04.03. | |
| 4,200 | 4,360 | 04.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 09.02. | Abeona Therapeutics Inc.: Abeona Therapeutics Announces New Employee Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 5 | GlobeNewswire (USA) | ||
| 31.12.25 | Abeona Therapeutics Inc.: Abeona Therapeutics Announces New Employee Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 3 | GlobeNewswire (USA) | ||
| 15.12.25 | Abeona Therapeutics beruft Mohamad Tabrizi zum Chief Business Officer | 7 | Investing.com Deutsch | ||
| ABEONA THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 15.12.25 | Abeona Therapeutics appoints Mohamad Tabrizi as chief business officer | 2 | Investing.com | ||
| 15.12.25 | Abeona Therapeutics Inc.: Abeona Therapeutics Announces Appointment of Mohamad Tabrizi as Chief Business Officer | 334 | GlobeNewswire (Europe) | CLEVELAND, Dec. 15, 2025 (GLOBE NEWSWIRE) -- Abeona Therapeutics Inc. (Nasdaq: ABEO) today announced the appointment of Mohamad Tabrizi, M.S., M.B.A., as Senior Vice President, Chief Business Officer... ► Artikel lesen | |
| 11.12.25 | Abeona Therapeutics Inc.: Abeona Therapeutics Announces New Qualified Treatment Center for ZEVASKYN in Texas | 2 | GlobeNewswire (USA) | ||
| 08.12.25 | Abeona Therapeutics Inc.: Abeona Therapeutics Announces First Patient Treatment with ZEVASKYN Gene Therapy | 284 | GlobeNewswire (Europe) | CLEVELAND, Dec. 08, 2025 (GLOBE NEWSWIRE) -- Abeona Therapeutics Inc. (Nasdaq: ABEO) today announced the first commercial patient treatment with FDA-approved ZEVASKYN (prademagene zamikeracel), a... ► Artikel lesen | |
| 01.12.25 | Abeona Therapeutics Inc.: Abeona Therapeutics Announces New Employee Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 3 | GlobeNewswire (USA) | ||
| 12.11.25 | Stifel lowers Abeona Therapeutics stock price target on launch delay | 18 | Investing.com | ||
| 12.11.25 | Abeona Therapeutics Shares Jump 21% On Strong Q3 Results | 11 | RTTNews | ||
| 12.11.25 | Here's Why Abeona Therapeutics Popped Higher Today | 20 | The Motley Fool | ||
| 12.11.25 | Abeona Therapeutics: EPS übertrifft Schätzungen um 0,17 $ - Umsatz schlechter als erwartet | 19 | Investing.com Deutsch | ||
| 12.11.25 | Abeona Therapeutics GAAP EPS of -$0.10 beats by $0.18 | 5 | Seeking Alpha | ||
| 12.11.25 | Abeona Therapeutics Inc.: Abeona Therapeutics Reports Third Quarter 2025 Financial Results and Corporate Updates | 358 | GlobeNewswire (Europe) | - First anticipated ZEVASKYN® patient treatment shifted to 4Q 2025 following implementation of optimized release assay - - Strong and growing patient demand and treatment site network, along with... ► Artikel lesen | |
| 12.11.25 | ABEONA THERAPEUTICS INC. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 12.11.25 | ABEONA THERAPEUTICS INC. - 8-K, Current Report | - | SEC Filings | ||
| 11.11.25 | What to Expect from Abeona Therapeutics' Earnings | 2 | Benzinga.com | ||
| 11.11.25 | Abeona Therapeutics Q3 2025 Earnings Preview | 3 | Seeking Alpha | ||
| 03.11.25 | Abeona Therapeutics Inc.: Abeona Therapeutics Announces New Employee Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 4 | GlobeNewswire (USA) | ||
| 30.10.25 | Abeona Therapeutics stock rises after receiving permanent J-code for ZEVASKYN | 9 | Investing.com |
1 2 3 Weiter >>
44 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 89,10 | -0,11 % | BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen | |
| EVOTEC | 5,652 | +6,56 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BB BIOTECH | 50,50 | +0,60 % | BB Biotech: Gleich mehrere Zukäufe - spannende News erwartet | Im Schlussquartal 2025 hat die Schweizer Biotechgesellschaft BB Biotech einige Veränderungen am Portfolio vorgenommen. Bereits kurz nach dem Kauf wurde bei zwei Werten bereits eine Übernahme angekündigt:... ► Artikel lesen | |
| MEDIGENE | 0,035 | -11,56 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 40,875 | +0,43 % | Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 49,585 | -0,17 % | Moderna: Kombi-Impfung für Grippe und Covid erhält Zulassung | Gute Nachrichten für Moderna: Die EU-Arzneimittelbehörde EMA hat grünes Licht gegeben für den ersten Kombi-Impfstoff gegen Corona und Grippe. Der Wirkstoff solle für Menschen ab 50 Jahren zugelassen... ► Artikel lesen | |
| PAION | 0,099 | +24,25 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,756 | +0,46 % | Valneva-Aktie: Die letzte Chance | Für den französischen Impfstoffentwickler Valneva entscheidet sich 2026 ein großer Teil der Investmentstory. Die Aktie wird derzeit fast ausschließlich durch die Erwartung der Phase-3-Daten zum Lyme-Borreliose-Impfstoff... ► Artikel lesen | |
| AMGEN | 326,85 | +0,31 % | Amgen EVP Sold $20.77M In Company Stock | ||
| EPIGENOMICS | 1,000 | +4,17 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,530 | +0,34 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 330,30 | +0,67 % | Jefferies reiterates Buy on Stryker stock, keeps $465 target | ||
| BIOGEN | 160,15 | +1,59 % | Biogen Inc.: The New England Journal of Medicine Publishes First Data to Demonstrate the Potential for Disease Modification in Dravet Syndrome | -Results show that targeting the underlying cause of Dravet syndrome with zorevunersen may improve outcomes for people with this rare, devastating genetic neurodevelopmental disease-
-Data support... ► Artikel lesen | |
| HEIDELBERG PHARMA | 3,020 | -2,58 % | HEIDELBERG PHARMA AG unter Beobachtung | ||
| ILLUMINA | 112,20 | +1,25 % | Evercore ISI reiterates Illumina stock rating on competitive positioning |
Das Unternehmen ABEONA THERAPEUTICS INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +1,40 % bei einem Aktienkurs von 4,340 Euro. Die Dividendendrendite beträgt aktuell 0,00 % (berechnet anhand der Ausschüttung von 0,00 Euro in den letzten 12 Monaten).
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ABEONA THERAPEUTICS INC finden Sie auf Wallstreet Online.