| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
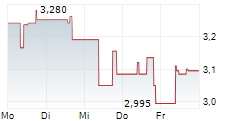
| 3,370 | 3,440 | 27.02. | |
| 3,360 | 3,420 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 17.02. | Jefferies startet Coverage für Cellectis mit Kaufempfehlung und hohem Kurspotenzial | 8 | Investing.com Deutsch | ||
| 17.02. | Jefferies assumes coverage on Cellectis stock with Buy rating | 4 | Investing.com | ||
| CELLECTIS Aktie jetzt für 0€ handeln | |||||
| 04.02. | Cellectis Inc.: Monthly information on share capital and company voting rights | 1 | GlobeNewswire (USA) | ||
| 08.01. | Cellectis Announces 2026 Strategy and Catalysts | 422 | GlobeNewswire (Europe) | NEW YORK, Jan. 08, 2026 (GLOBE NEWSWIRE) -- Cellectis (the "Company") (Euronext Growth: ALCLS - NASDAQ: CLLS), a clinical-stage biotechnology company using its pioneering gene editing platform to... ► Artikel lesen | |
| 08.01. | Cellectis S.A. - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 05.01. | Cellectis Inc.: Monthly information on share capital and company voting rights | 4 | GlobeNewswire (USA) | ||
| 22.12.25 | Clear Street initiates Cellectis stock with Buy rating on CAR-T potential | 12 | Investing.com | ||
| 16.12.25 | Allogene Arbitration Victory Pressures Cellectis Shares Tuesday | 11 | Benzinga.com | ||
| 15.12.25 | Allogene Therapeutics, Inc.: Allogene Therapeutics Reports Favorable Result for Servier in Arbitration with Cellectis | 218 | GlobeNewswire (Europe) | Arbitration Ruling Reaffirms Allogene's Full Control of Cemacabtagene Ansegedleucel (Cema-Cel)Decision Reconfirms Allogene's Expanded Sub-License Covering EU and UK Rights with Options for Japan and... ► Artikel lesen | |
| 15.12.25 | Schiedsspruch beendet Lizenzvereinbarung zwischen Cellectis und Servier teilweise | 16 | Investing.com Deutsch | ||
| 15.12.25 | Arbitration ruling partially terminates Cellectis-Servier license agreement | 1 | Investing.com | ||
| 15.12.25 | Cellectis Announces Arbitral Decision in Dispute with Servier | 445 | GlobeNewswire (Europe) | NEW YORK, Dec. 15, 2025 (GLOBE NEWSWIRE) -- Cellectis (the "Company") (Euronext Growth: ALCLS - NASDAQ: CLLS), a clinical-stage biotechnology company using its pioneering gene-editing platform to... ► Artikel lesen | |
| 15.12.25 | Cellectis S.A. - 6-K, Report of foreign issuer | 5 | SEC Filings | ||
| 08.12.25 | Cellectis Inc.: ASH 2025: Cellectis Presents Development Plan to Further Enhance High Response Rate Observed for Eti-cel in r/r NHL | 5 | GlobeNewswire (USA) | ||
| 05.12.25 | Cellectis S.A. - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 03.12.25 | Cellectis Inc.: Monthly information on share capital and company voting rights | 4 | GlobeNewswire (USA) | ||
| 21.11.25 | Moligo and Cellectis show promise in non-viral gene editing method | 3 | Investing.com | ||
| 19.11.25 | Why Cellectis Stock Is Trading Higher Today | 4 | Benzinga.com | ||
| 19.11.25 | Cellectis reports breakthrough in non-viral gene editing technique | 11 | Investing.com | ||
| 19.11.25 | Cellectis Publishes Nature Communications Article on a Non-Viral Gene Editing Process Enabling Efficient Gene Insertion in Hematopoietic Stem Cells | 1 | GlobeNewswire (USA) |
1 2 3 Weiter >>
50 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
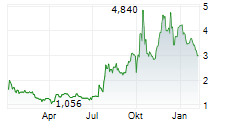
CELLECTIS SA gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell +0,30 % bei einem Aktienkurs von 3,400 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CELLECTIS SA finden Sie auf Wallstreet Online.