- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 17.02. | Cingulate Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 06.02. | Cingulate Inc. - 8-K, Current Report | 8 | SEC Filings | ||
| 28.01. | Cingulate Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 15.01. | Cingulate weitet Kapitalbeschaffungsprogramm auf 31,9 Mio. $ aus | 7 | Investing.com Deutsch | ||
| 15.01. | Cingulate Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| CINGULATE Aktie jetzt für 0€ handeln | |||||
| 02.01. | Cingulate Inc. - 8-K, Current Report | 4 | SEC Filings | ||
| 15.12.25 | Cingulate Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 13.11.25 | Cingulate Inc. - 10-Q, Quarterly Report | 7 | SEC Filings | ||
| 13.11.25 | Cingulate reports Q3 results | 2 | Seeking Alpha | ||
| 13.11.25 | Cingulate Inc. Reports Third Quarter 2025 Financial Results and Provides Corporate Update | 259 | GlobeNewswire (Europe) | Industry Veteran Bryan Downey Named Chief Commercial Officer NDA Accepted with May 2026 PDUFA Date Commercial Supply Agreement Executed KANSAS CITY, Kan., Nov. 13, 2025 (GLOBE NEWSWIRE) -- Cingulate... ► Artikel lesen | |
| 10.11.25 | Cingulate Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 23.10.25 | Cingulate Inc.: Cingulate's Lead ADHD Candidate CTx-1301 Selected for Podium Presentation at AACAP Annual Meeting | 11 | GlobeNewswire (USA) | ||
| 19.10.25 | This analyst just raised his price target on Cingulate | 9 | Cantech Letter | ||
| 14.10.25 | FDA Accepts Cingulate's NDA For ADHD Candidate CTx-1301 | 10 | RTTNews | ||
| 14.10.25 | FDA accepts Cingulate's ADHD drug application, sets May 2026 target date | 4 | Investing.com | ||
| 14.10.25 | Cingulate Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 25.09.25 | Cingulate Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 19.09.25 | Should you sell your Cingulate stock? | 5 | Cantech Letter | ||
| 17.09.25 | Cingulate secures manufacturing deal with Bend Bio Sciences for ADHD drug | 5 | Investing.com | ||
| 17.09.25 | Cingulate Inc.: Cingulate Secures Exclusive Manufacturing Partnership with Bend Bio Sciences to Support Future Commercialization of Next Generation ADHD Treatment | 7 | GlobeNewswire (USA) |
1 2 Weiter >>
26 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| ILLUMINA | 113,72 | -0,09 % | Evercore ISI reiterates Illumina stock rating on competitive positioning |
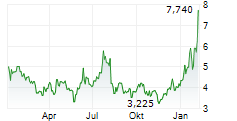
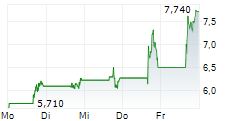
CINGULATE INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,215 (-3,31 %) liegt der Kurs bei 6,290 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CINGULATE INC finden Sie auf Wallstreet Online.