| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
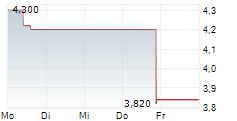
| 3,760 | 3,820 | 14:57 | |
| 3,760 | 3,800 | 14:54 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Cytek Biosciences, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 2 | SEC Filings | ||
| Fr | Cytek Biosciences outlines $205M-$212M 2026 revenue target amid record Q4 and growth in recurring revenue | 2 | Seeking Alpha | ||
| Do | Cytek Biosciences: Rekordumsatz im vierten Quartal, Gewinnziel jedoch deutlich verfehlt | 1 | Investing.com Deutsch | ||
| CYTEK BIOSCIENCES Aktie jetzt für 0€ handeln | |||||
| Do | Cytek Biosciences GAAP EPS of -$0.34 misses by $0.30, revenue of $62.1M beats by $3.3M | 1 | Seeking Alpha | ||
| Do | Cytek Biosciences, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| Do | Cytek Biosciences, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 12.02. | Cytek Biosciences, Inc.: Cytek Biosciences to Report Fourth Quarter and Full Year 2025 Financial Results on February 26, 2026 | 1 | GlobeNewswire (USA) | ||
| 13.01. | Cytek Biosciences, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 12.01. | Cytek Biosciences Preliminary Q4 Revenue Up 8% | 1 | RTTNews | ||
| 12.01. | Cytek Biosciences, Inc.: Cytek Biosciences Announces Preliminary Fourth Quarter and Full Year 2025 Revenue Results | 101 | GlobeNewswire (Europe) | FREMONT, Calif., Jan. 12, 2026 (GLOBE NEWSWIRE) -- Cytek Biosciences, Inc. (Nasdaq: CTKB), a leading cell analysis solutions company, today announced preliminary, unaudited revenue results for the... ► Artikel lesen | |
| 12.11.25 | Cytek Biosciences, Inc.: TIME Recognizes Cytek Biosciences as One of America's Growth Leaders of 2026 | 5 | GlobeNewswire (USA) | ||
| 06.11.25 | Cytek Biosciences, Inc.: Cytek Muse Micro Cell Analyzer Wins BioTech Breakthrough Award for Drug Discovery Solution of the Year | 4 | GlobeNewswire (USA) | ||
| 06.11.25 | Cytek reaffirms $196M-$205M 2025 revenue outlook as recurring businesses accelerate | 3 | Seeking Alpha | ||
| 05.11.25 | Cytek Biosciences GAAP EPS of -$0.04 beats by $0.01, revenue of $52.3M beats by $1.16M | 5 | Seeking Alpha | ||
| 05.11.25 | Cytek Biosciences, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 29.10.25 | Cytek Biosciences, Inc.: Cytek Biosciences Deepens Commitment to Expanding Access to Flow Cytometry | 1.195 | GlobeNewswire (Europe) | FREMONT, Calif., Oct. 29, 2025 (GLOBE NEWSWIRE) -- Amid the reduction of various government grant programs and global economic challenges that have tightened research funding, Cytek Biosciences, Inc.... ► Artikel lesen | |
| 22.10.25 | Cytek Biosciences, Inc.: Cytek Biosciences to Report Third Quarter 2025 Financial Results on November 5, 2025 | 2 | GlobeNewswire (USA) | ||
| 16.10.25 | Cytek Biosciences Expands with New Facility in Amsterdam | 3 | Contract Pharma | ||
| 15.10.25 | Cytek Biosciences, Inc.: Cytek Biosciences Expands European Presence with New Facility in Amsterdam to Better Serve Customers and Drive Growth | 1 | GlobeNewswire (USA) | ||
| 18.03.25 | Cytek Biosciences, Inc.: Cytek Biosciences Expands Cell Analysis Offerings with the Affordable, User-Friendly Cytek Muse Micro System | 461 | GlobeNewswire (Europe) | FREMONT, Calif., March 18, 2025 (GLOBE NEWSWIRE) -- Today, Cytek Biosciences, Inc. (Nasdaq: CTKB) announced that it has added transformative capabilities to its iconic Cytek® Guava® Muse® cell analyzer... ► Artikel lesen |
20 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| MEDIGENE | 0,041 | +1,50 % | Medigene: Zurückziehung - 18.11.2025 | ||
| BIOFRONTERA | 2,690 | +0,37 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| CLINUVEL | 6,180 | +2,83 % | Clinuvel Pharmaceuticals Limited H1 Profit Declines | ||
| ASEP MEDICAL | 0,121 | 0,00 % | ASEP Medical Holdings Inc (2): Asep Medical appoints Heinzl as interim CEO | ||
| CELLECTAR BIOSCIENCES | 3,290 | 0,00 % | Cellectar Biosciences, Inc.: Cellectar Biosciences Announces Strategic Supply Agreement with Ionetix for Actinium-225 and Astatine-211 to Advance Targeted Alpha Therapies | FLORHAM PARK, N.J. and LANSING, Mich., Dec. 16, 2025 (GLOBE NEWSWIRE) -- Cellectar Biosciences, Inc. (NASDAQ: CLRB), a late-stage clinical biopharmaceutical company focused on the discovery and development... ► Artikel lesen | |
| CARDIFF ONCOLOGY | 1,496 | -8,67 % | Cardiff Oncology, Inc.: Cardiff Oncology Reports Full Year 2025 Results and Provides Business Update | Reported positive update from Phase 2 CRDF-004 trial in first-line RAS-mutated mCRC, with the 30 mg onvansertib + FOLFIRI/bev arm demonstrating:• Robust ORR of 72.2% (vs 43.2% with combined SoC of... ► Artikel lesen | |
| CLEAN HARBORS | 248,00 | -0,08 % | Clean Harbors Stock Barely Moves Despite Q4 Earnings and Revenue Beat | ||
| LIR LIFE SCIENCES | 0,604 | -0,66 % | EQS-Media: First-Mover mit erheblichem Skalierungspotenzial: LIR Life Sciences setzt Innovations-Roadmap fort | EQS-Media / 06.02.2026 / 16:20 CET/CEST
LIR Life Sciences Corp. (ISIN: CA50206C1005 | WKN: A41QA9), LIR oder das Unternehmen, freut sich bekannt zu geben, dass es mit "Neuland Laboratories Limited"... ► Artikel lesen | |
| MESOBLAST | 1,230 | -8,21 % | Biotech vor dem Comeback? Was bei Bayer, Vidac und Mesoblast jetzt zählt | ||
| SPERO THERAPEUTICS | 1,868 | +1,41 % | Anterix Inc.: Anterix Appoints Ross Spero as Chief Product Officer | WOODLAND PARK, N.J., Jan. 07, 2026 (GLOBE NEWSWIRE) -- Anterix, Inc. (NASDAQ: ATEX) today announced the appointment of Ross Spero as Chief Product Officer. Spero will lead Anterix's product development... ► Artikel lesen | |
| CSTONE PHARMACEUTICALS | 0,720 | +0,70 % | CStone Secures MHRA Approval For Sugemalimab In Stage III NSCLC In UK | ||
| PROTALIX BIOTHERAPEUTICS | 2,480 | +0,81 % | Chiesi Global Rare Diseases and Protalix BioTherapeutics Receive Positive CHMP Opinion for an Additional Dosing Regimen of Every Four Weeks for Elfabrio (pegunigalsidase alfa) in the EU | Committee
for
Medicinal
Products
for
Human
Use
(CHMP)
issues
a
positive
opinion following re-examination, which
will
be reviewed by
the... ► Artikel lesen | |
| PALVELLA THERAPEUTICS | 116,00 | +0,87 % | Palvella Therapeutics Inc.: Palvella Therapeutics Announces Closing of Upsized Public Offering of Common Stock and Exercise in Full of the Underwriters' Option to Purchase Additional Shares | WAYNE, Pa., March 02, 2026 (GLOBE NEWSWIRE) -- Palvella Therapeutics, Inc. ("Palvella") (Nasdaq: PVLA), a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapies... ► Artikel lesen | |
| PURPLE BIOTECH | - | - | XFRA CAPITAL ADJUSTMENT INFORMATION - 27.02.2026 | Das Instrument UZ9 CA4577022078 INSPIRATION ENERGY CORP. EQUITY wird cum Kapitalmassnahme gehandelt am 27.02.2026 und ex Kapitalmassnahme am 02.03.2026 The instrument UZ9 CA4577022078 INSPIRATION ENERGY... ► Artikel lesen |
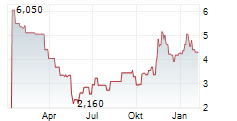
Das Unternehmen CYTEK BIOSCIENCES INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell -5,79 % bei einem Aktienkurs von 3,580 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CYTEK BIOSCIENCES INC finden Sie auf Wallstreet Online.