- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 17:38 | Egetis Therapeutics Launches Educational Websites on MCT8 Deficiency for Physicians and Caregivers | 180 | ACCESS Newswire | STOCKHOLM, SWEDEN / ACCESS Newswire / March 2, 2026 / Egetis Therapeutics AB (publ) ("Egetis" or the "Company") (NASDAQ Stockholm:EGTX), today announced the launch of two updated educational websites... ► Artikel lesen | |
| Do | Egetis Therapeutics AB: Year-End Report January-December 2025 | 79 | GlobeNewswire (Europe) | Egetis submitted the U.S. NDA for Emcitate® (tiratricol) for treatment of MCT8 deficiency· Emcitate® revenue during 2025 amounted to MSEK 62.3, a 40 % increase in constant exchange rates from... ► Artikel lesen | |
| 16.02. | Egetis Therapeutics AB: Egetis provides update on progress with the development of Emcitate (tiratricol) for MCT8 deficiency in Japan | 135 | GlobeNewswire (Europe) | Stockholm, Sweden, February 16, 2026. Egetis Therapeutics AB (publ) ("Egetis" or the "Company") (NASDAQ Stockholm: EGTX), today announced that its Japanese partner Fujimoto Pharmaceuticals, who has... ► Artikel lesen | |
| EGETIS THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 04.02. | Egetis Therapeutics AB: FDA's rare pediatric disease priority review program extended until 2029 | 168 | GlobeNewswire (Europe) | Stockholm, Sweden, February 4, 2026. Egetis Therapeutics AB (publ) ("Egetis" or the "Company") (NASDAQ Stockholm: EGTX), today noted that the U.S. Food and Drug Administration's (FDA's) Rare Pediatric... ► Artikel lesen | |
| 29.01. | Egetis Therapeutics AB: Egetis Completes the U.S. Rolling NDA Submission for Emcitate (tiratricol) for Treatment of MCT8 Deficiency | 106 | GlobeNewswire (Europe) | Priority Review and Rare Pediatric Disease Priority Review Voucher requestedAnticipated regulatory decision in September 2026Emcitate® (tiratricol) U.S. launch expected in Q4 2026, if approvedStockholm... ► Artikel lesen | |
| 19.12.25 | Egetis Therapeutics AB: Egetis Therapeutics initiates New Drug Application in the USA for Emcitate (tiratricol) for MCT8 deficiency | 225 | GlobeNewswire (Europe) | Stockholm, Sweden, December 19, 2025. Egetis Therapeutics AB (publ) ("Egetis" or the "Company") (NASDAQ Stockholm: EGTX), today announced that the Company has initiated a rolling New Drug Application... ► Artikel lesen | |
| 10.12.25 | Egetis Therapeutics AB: Egetis and Er-Kim Expand Partnership to Broaden Access to Emcitate Across Central, Eastern, and Southeastern Europe | 141 | GlobeNewswire (Europe) | Stockholm, Sweden, December 10, 2025. Egetis Therapeutics AB (publ) ("Egetis" or the "Company") (NASDAQ Stockholm: EGTX), today announced that the Company, through its wholly-owned subsidiary Rare Thyroid... ► Artikel lesen | |
| 25.11.25 | Egetis-Aktie unter Druck: Positive Studiendaten können Kursverlust nach Q3-Zahlen nicht verhindern | 4 | Investing.com Deutsch | ||
| 25.11.25 | Egetis Therapeutics AB: Interim report Q3 2025 | 167 | GlobeNewswire (Europe) | Egetis to commence a rolling NDA submission in December as granted by the FDA for Emcitate® (tiratricol)· Egetis received FDA Breakthrough Therapy Designation for Emcitate® (tiratricol) for... ► Artikel lesen | |
| 14.11.25 | Egetis Therapeutics AB: Egetis announces positive results from the ReTRIACt study of Emcitate (tiratricol) in MCT8 deficiency | 138 | GlobeNewswire (Europe) | Positive topline results demonstrate a statistically significant (p=0.034) difference in the rate of change in serum T3 in patients randomized to withdrawal (placebo) vs. in patients continuing therapy... ► Artikel lesen | |
| 23.10.25 | Egetis Therapeutics AB: Egetis granted a rolling NDA review by FDA for Emcitate (tiratricol) based on currently available clinical data | 226 | GlobeNewswire (Europe) | The outcomes of a successful pre-NDA meeting with the FDA are:
As agreed with the FDA, the rolling NDA submission will commence in December 2025, targeting a complete NDA submission in early 2026 and... ► Artikel lesen | |
| 02.10.25 | Egetis Therapeutics AB: Egetis Therapeutics intends to carry out a directed share issue of up to 10 percent of outstanding ordinary shares | 190 | GlobeNewswire (Europe) | NOT FOR PUBLICATION, DISTRIBUTION OR RELEASE, DIRECTLY OR INDIRECTLY, IN WHOLE OR IN PART, IN OR INTO THE UNITED STATES OF AMERICA, AUSTRALIA, CANADA, HONG KONG, ISRAEL, JAPAN, NEW ZEALAND, SOUTH AFRICA... ► Artikel lesen | |
| 21.08.25 | Egetis Therapeutics AB: Interim report Q2 2025 | 243 | GlobeNewswire (Europe) | Egetis reports progress towards US NDA submission for tiratricol· FDA awarded tiratricol Breakthrough Therapy Designation (BTD) in July 2025, based on the Agency's review of Egetis' analysis... ► Artikel lesen | |
| 18.08.25 | Egetis Therapeutics AB: Egetis reports progress towards US NDA submission for tiratricol | 520 | GlobeNewswire (Europe) | FDA awarded tiratricol Breakthrough Therapy Designation (BTD) in July 2025, based on the Agency's review of Egetis' analysis of the survival data set from the international real-world cohort study by... ► Artikel lesen | |
| 15.07.25 | Egetis Therapeutics AB: Egetis receives FDA Breakthrough Therapy Designation for tiratricol for MCT8 deficiency | 343 | GlobeNewswire (Europe) | Stockholm, Sweden, July 15, 2025. Egetis Therapeutics AB (publ) ("Egetis" or the "Company") (NASDAQ Stockholm: EGTX), today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough... ► Artikel lesen | |
| 05.05.25 | Egetis Therapeutics AB: Egetis Confirms Launch of Emcitate in Germany | 518 | GlobeNewswire (Europe) | For investors and media only
Stockholm, Sweden, May 5, 2025. Egetis Therapeutics AB (publ) ("Egetis" or the "Company") (NASDAQ Stockholm: EGTX), a pharmaceutical company dedicated to developing therapies... ► Artikel lesen | |
| 30.04.25 | Egetis Therapeutics AB: Interim report Q1 2025 | 218 | GlobeNewswire (Europe) | Egetis plans to launch Emcitate® in the first country, Germany, on May 1, 2025The European Commission approved Emcitate® (tiratricol) as the first and only treatment for patients with MCT8 deficiency.In... ► Artikel lesen |
17 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| SANOFI | 81,51 | -0,28 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,930 | +0,48 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ASTRAZENECA | 174,95 | -0,85 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TILRAY BRANDS | 6,560 | -1,80 % | MDMA-Herausforderer Bioxyne punktet mit 112% Umsatzplus. Und was macht Tilray? | ||
| DERMAPHARM | 39,650 | +0,89 % | Dermapharm: Spannend für langfristig orientierte Investoren | Am 17. März wird Dermapharm Zahlen für 2025 präsentieren. Erwartet wird von Dermapharm ein Umsatz von 1,16 Milliarden Euro bis 1,2 Milliarden Euro. Das bereinigte EBITDA sieht die Gesellschaft bei 322... ► Artikel lesen | |
| QUANTUM BIOPHARMA | 3,080 | +1,99 % | Quantum Biopharma Announces Completion of Dosing in 180-Day Repeated Dose Oral Toxicity and Toxicokinetic Studies for Lucid-21-302 (Lucid-MS) | TORONTO, Dec. 23, 2025 (GLOBE NEWSWIRE) -- Quantum BioPharma Ltd. (NASDAQ: QNTM) (CSE: QNTM) (FRA: 0K91) (Upstream: QNTM) ("Quantum BioPharma" or the "Company"), a biopharmaceutical company dedicated... ► Artikel lesen | |
| TONIX PHARMACEUTICALS | 11,900 | 0,00 % | Tonix Pharmaceuticals Holding Corp.: Tonix Pharmaceuticals Announces Program Updates on Phase 2/3-Ready Long-Acting Monoclonal Antibody (mAb) Designed for Seasonal Prevention of Lyme Disease (TNX-4800) | Exploring clinical development plan options including a controlled human infection model (CHIM) and a Phase 2/3 adaptive field study Expect to have investigational product of TNX-4800 (anti-Borrelia... ► Artikel lesen | |
| CASSAVA SCIENCES | 1,951 | +0,91 % | Cassava Sciences, Inc.: Cassava Announces Agreement to Settle Securities Class Action Litigation | AUSTIN, Texas, Dec. 23, 2025 (GLOBE NEWSWIRE) -- Cassava Sciences, Inc. (NASDAQ: SAVA, "Cassava", the "Company"), a biotechnology company focused on developing novel, investigational treatments for... ► Artikel lesen | |
| OPKO HEALTH | 1,050 | +2,92 % | OPKO Health, Inc.: OPKO Health Reports Fourth Quarter 2025 Business Highlights and Financial Results | MIAMI, Feb. 26, 2026 (GLOBE NEWSWIRE) -- OPKO Health, Inc. (NASDAQ: OPK) (OPKO) reports business highlights and financial results for the three and 12 months ended December 31, 2025, and introduces... ► Artikel lesen | |
| SELLAS LIFE SCIENCES | 4,395 | +5,14 % | SELLAS LIFE SCIENCES GROUP INC zeigt strukturelle Stärke | ||
| SOLIGENIX | 1,140 | -3,39 % | Soligenix Gets Positive EMA Opinion For Orphan Drug Status Of SGX945 | ||
| BETTERLIFE PHARMA | 0,031 | -8,96 % | Betterlife Pharma Inc (2): Betterlife Pharma grants options to buy 6.05M shares | ||
| THERIVA BIOLOGICS | 0,199 | +1,64 % | Weekly Buzz: NRXP Sets The Path For NRX-100's NDA; IRON Gets FDA's CRL; TOVX Licenses SYN-020 | NEW BRUNSWICK (dpa-AFX) - The biotech space this week witnessed significant key milestones, including FDA approvals, NDA path-setting meetings, rejections, licensing agreements, and oncology... ► Artikel lesen | |
| NOVABAY PHARMACEUTICALS | 1,515 | +6,69 % | XFRA NEW INSTRUMENTS AVAILABLE ON 23.02.2026 | The following instruments on XETRA do have their first trading 23.02.2026 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 23.02.2026
Aktien
1 CA43708V1067 The Home Depot Inc.... ► Artikel lesen | |
| BAYER | 40,610 | -2,95 % | Aktien von Rheinmetall, Infineon, Bayer & Co. sind es nicht! ... und wie Börsenweisheiten über das schwierige Jahr 2025 hinweghelfen konnten. | Guten Tag, liebe Leserinnen und Leser,
viele von Ihnen kennen sie wahrscheinlich gar nicht mal. Aber vielleicht ist Ihnen doch schon die ein oder andere Börsenweisheit untergekommen, die aufgrund ihrer... ► Artikel lesen |
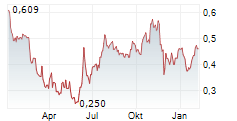
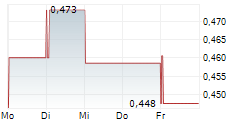
Das Unternehmen EGETIS THERAPEUTICS AB kann der Branche Pharma zugeordnet werden. Der aktuelle Kurs liegt bei 0,399 Euro und damit +4,59 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu EGETIS THERAPEUTICS AB finden Sie auf Wallstreet Online.