| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 4,270 | 4,360 | 18:08 | |
| 4,270 | 4,360 | 18:08 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 14.01. | SELLAS partners with IMPACT-AML for European SLS009 trials | 27 | Investing.com | ||
| 14.01. | SELLAS Life Sciences Group, Inc.: SELLAS Life Sciences Enters Agreement with IMPACT-AML to Expand SLS009 Clinical Program into Europe | 15 | GlobeNewswire (USA) | ||
| 08.01. | SELLAS Life Sciences Group, Inc. - 8-K, Current Report | 19 | SEC Filings | ||
| 29.12.25 | Update zu Leukämie-Studie beflügelt Aktie von Sellas Life Sciences | 67 | Investing.com Deutsch | ||
| 29.12.25 | SELLAS meldet 72 Ereignisse in Phase-3-Studie REGAL und wartet auf finale Analyse | 47 | Investing.com Deutsch | ||
| 29.12.25 | SELLAS reports 72 events in Phase 3 REGAL trial, awaits final analysis | 16 | Investing.com | ||
| 29.12.25 | SELLAS Life Sciences Group, Inc.: SELLAS Life Sciences Provides Update on Pivotal Phase 3 REGAL Trial of Galinpepimut-S (GPS) in Acute Myeloid Leukemia (AML) | 1.148 | GlobeNewswire (Europe) | Contract Research Organization for the REGAL trial has informed the Company that 72 events have occurred in the trial as of December 26, 2025; SELLAS remains blinded to trial outcomesTiming of the... ► Artikel lesen | |
| 08.12.25 | Sellas Life Sciences: Aktie legt nach vielversprechenden Studiendaten zu AML-Therapie zu | 44 | Investing.com Deutsch | ||
| SELLAS LIFE SCIENCES Aktie jetzt für 0€ handeln | |||||
| 08.12.25 | SELLAS Rises On Positive Phase 2 Data Of SLS009 In Relapsed/Refractory AML-MR | 6 | RTTNews | ||
| 08.12.25 | SELLAS Life Sciences Group, Inc.: SELLAS Life Sciences Presents Positive Phase 2 Data of SLS009 in Combination with AZA/VEN in Relapsed/Refractory AML-MR at ASH 2025 | 6 | GlobeNewswire (USA) | ||
| 13.11.25 | SELLAS Life Sciences GAAP EPS of -$0.06 beats by $0.02 | 9 | Seeking Alpha | ||
| 12.11.25 | SELLAS Life Sciences Group, Inc. - 10-Q, Quarterly Report | 5 | SEC Filings | ||
| 03.11.25 | SELLAS präsentiert Daten zu SLS009 bei rezidivierter Leukämie auf ASH-Tagung | 23 | Investing.com Deutsch | ||
| 03.11.25 | SELLAS to present SLS009 data for relapsed leukemia at ASH meeting | 10 | Investing.com | ||
| 27.10.25 | SELLAS Life Sciences raises $31 million through warrant exercise | 16 | Investing.com | ||
| 27.10.25 | SELLAS Life Sciences Group, Inc.: SELLAS Life Sciences Group Announces Exercise of Existing Warrants Held by a Current Institutional Investor for $31 Million in Gross Proceeds | 3 | GlobeNewswire (USA) | ||
| 27.10.25 | SELLAS Life Sciences Group, Inc. - 8-K, Current Report | 6 | SEC Filings | ||
| 13.10.25 | SELLAS to present CDK9 inhibitor data for T-cell leukemia at ESMO | 8 | Investing.com | ||
| 13.10.25 | SELLAS Life Sciences Group, Inc.: SELLAS Life Sciences to Present In Vivo Preclinical Data Demonstrating Statistically Significant Survival Benefit of SLS009 in T-Cell Prolymphocytic Leukemia at the European Society for Medical Oncology (ESMO) ... | 9 | GlobeNewswire (USA) | ||
| 10.10.25 | Sellas Life Sciences files to sell 19.69M shares of common stock for holders | 9 | Seeking Alpha |
1 2 Weiter >>
33 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 38,310 | -5,66 % | Bayer auf dem Prüfstand: Was die Lösung des Glyphosat-Streits für die Aktie bedeutet | Bayer hofft auf eine Lösung des Glyphosat-Rechtsstreits bis 2026. Was bedeutet das für die Aktie? Bayer hat in den verganngenen zwölf Monaten eine beeindruckende Aktienperformance von rund 90 Prozent... ► Artikel lesen | |
| NOVO NORDISK | 31,325 | -2,63 % | maydornsmeinung: Silber, Nvidia, AMD, Microsoft, IBM, SAP, TeamViewer, Novo Nordisk, Nebius & Co. | In maydornsmeinung geht es zunächst um den Gesamtmarkt: Trotz Zoll-Chaos bleiben für Alfred die Unternehmensgewinne der entscheidende Faktor. Die Berichtssaison läuft stark, auch wenn sich die großen... ► Artikel lesen | |
| PFIZER | 22,945 | -1,54 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 139,66 | -2,36 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 121,35 | -3,88 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 126,72 | -1,26 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,075 | -3,60 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 79,71 | -2,22 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,350 | -2,33 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 200,00 | -0,25 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 392,65 | -1,11 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,908 | -2,26 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 866,00 | -0,52 % | Aktien New York Ausblick: Stabile Kurse vor Rede von Trump - AMD-Aktie stark | NEW YORK (dpa-AFX) - Nach dem sehr schwachen Wochenauftakt stehen die Vorzeichen für die US-Börsen am Dienstag auf Stabilisierung. Größere Sprünge nach oben sind aber nicht zu erwarten. Stattdessen... ► Artikel lesen | |
| MERCK & CO | 103,40 | -0,39 % | Merck to collaborate with Tempus AI on precision oncology |
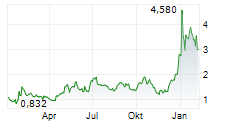
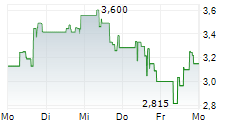
Das Unternehmen SELLAS LIFE SCIENCES GROUP INC kann der Branche Pharma zugeordnet werden. Mit einer aktuellen Kursveränderung von -0,060 (-1,39 %) liegt der Kurs bei 4,265 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SELLAS LIFE SCIENCES GROUP INC finden Sie auf Wallstreet Online.