| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
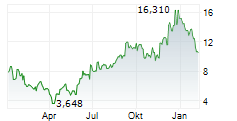
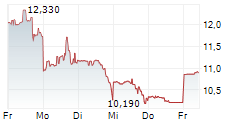
| 14,635 | 14,750 | 13:03 | |
| 14,475 | 15,220 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | EyePoint Pharmaceuticals, Inc.: EyePoint to Report Fourth Quarter and Full-Year 2025 Financial Results on March 4, 2026 | 3 | GlobeNewswire (USA) | ||
| 18.02. | EyePoint Pharmaceuticals, Inc.: EyePoint Appoints Michael Campbell as Chief Commercial Officer | 520 | GlobeNewswire (Europe) | - Seasoned commercial executive with over 30 years of leadership in retinal disease across biotech and large pharma -- Brings established track record of successful product launches and oversight... ► Artikel lesen | |
| 17.02. | Guggenheim reiterates EyePoint stock rating on rival trial data | 17 | Investing.com | ||
| EYEPOINT Aktie jetzt für 0€ handeln | |||||
| 06.02. | RBC Capital reiterates Outperform rating on EyePoint stock amid recent decline | 9 | Investing.com | ||
| 02.02. | Mizuho reiterates Outperform rating on EyePoint stock amid share weakness | 5 | Investing.com | ||
| 16.01. | EyePoint Pharmaceuticals, Inc.: EyePoint Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4) | 6 | GlobeNewswire (USA) | ||
| 07.01. | EyePoint: Mizuho bekräftigt "Outperform"-Rating und Kursziel von 33 $ | 11 | Investing.com Deutsch | ||
| 07.01. | Mizuho reiterates Outperform rating on EyePoint stock with $33 target | 1 | Investing.com | ||
| 07.01. | EyePoint's Phase 3 trials for DURAVYU in wet AMD on track for mid-2026 data | 1 | Investing.com | ||
| 07.01. | EyePoint: Phase-3-Studiendaten für DURAVYU bei feuchter AMD für Mitte 2026 erwartet | 3 | Investing.com Deutsch | ||
| 07.01. | EyePoint Pharmaceuticals, Inc.: EyePoint Reports Corporate Update and Anticipated Pivotal Milestones for 2026 | 651 | GlobeNewswire (Europe) | - Phase 3 programs underway for DURAVYU in wet AMD and DME, the largest multi-billion-dollar retinal disease markets - - Pivotal Phase 3 trials in wet AMD on track for data readout beginning in mid-2026... ► Artikel lesen | |
| 07.01. | EyePoint, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 16.12.25 | EyePoint Pharmaceuticals, Inc.: EyePoint Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4) | 8 | GlobeNewswire (USA) | ||
| 09.12.25 | EyePoint stock maintains Outperform rating at Mizuho amid competitor moves | 8 | Investing.com | ||
| 09.12.25 | Mizuho bestätigt "Outperform"-Rating für EyePoint trotz beschleunigter Konkurrenz-Pläne | 18 | Investing.com Deutsch | ||
| 08.12.25 | EyePoint Pharmaceuticals ändert Namen, Kürzel EYPT bleibt bestehen | 10 | Investing.com Deutsch | ||
| 08.12.25 | EyePoint Pharmaceuticals, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 20.11.25 | EyePoint Pharmaceuticals stock maintains Buy rating at H.C. Wainwright | 6 | Investing.com | ||
| 19.11.25 | EyePoint Pharmaceuticals, Inc.: EyePoint Announces Positive Recommendation from Independent Data Safety Monitoring Committee for Pivotal Phase 3 Trials for DURAVYU in Wet Age-Related Macular Degeneration | 289 | GlobeNewswire (Europe) | - No changes in protocol recommended for LUGANO and LUCIA clinical trials - - Masked safety data continues to show no safety signals, consistent with previous clinical trials for DURAVYU - - On track... ► Artikel lesen | |
| 19.11.25 | EyePoint Pharmaceuticals, Inc. - 8-K, Current Report | - | SEC Filings |
1 2 3 Weiter >>
53 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 42,010 | +0,39 % | Märkte am Morgen: Bayer, SAP, Scout24, Knorr-Bremse, thyssenkrupp, Bitcoin | Der DAX hat eine turbulente Woche hinter sich. Am Freitag gab es noch einmal einen ordentlichen Schub nach oben. In den USA hatte der Supreme Court die Zölle von Donald Trump für unrechtmäßig erklärt.... ► Artikel lesen | |
| NOVO NORDISK | 31,600 | -0,24 % | Opening Bell: Coca-Cola, Nvidia, Eli Lilly, Novo Nordisk, Vistra Energy, Energy Fuels | An der Wall Street richtet sich die Aufmerksamkeit mal wieder auf die Zollpolitik von Donald Trump. Der Supreme Court hatte am Freitag die bisherigen Maßnahmen gekippt. Trump mit neuen Zöllen unter... ► Artikel lesen | |
| PFIZER | 23,350 | -0,17 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| NOVARTIS | 142,44 | +0,21 % | dpa-AFX-Überblick: UNTERNEHMEN - Die wichtigsten Meldungen vom Wochenende | GESAMT-ROUNDUP 2: Kommt eine Social-Media-Altersgrenze ab 14 Jahren? STUTTGART - Ein Mindestalter für soziale Medien wie Tiktok und Instagram zum Schutz von Kindern und Jugendlichen rückt immer näher.... ► Artikel lesen | |
| MERCK KGAA | 128,60 | +2,35 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 125,66 | -0,33 % | Gilead Sciences schlägt zu - 68 Prozent Aufschlag | Die US-amerikanische Biotech-Gesellschaft Gilead Sciences will sich im Bereich der Onkologie verstärken. Mit der geplanten Übernahme von Arcellx erwirbt das Unternehmen die volle Kontrolle über eine... ► Artikel lesen | |
| AURORA CANNABIS | 3,240 | -1,22 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,41 | -0,40 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 25,040 | +0,93 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 196,00 | -0,31 % | Rekordquartal und AbbVie-Deal beflügeln Gubra-Aktie: Kursplus von 13,88 % | ||
| ROCHE | 402,50 | -0,27 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,950 | +0,85 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 889,90 | -0,11 % | Novo posts worst monthly decline as Lilly extends lead | ||
| MERCK & CO | 104,60 | -0,19 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen |
EYEPOINT INC gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 14,670 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu EYEPOINT INC finden Sie auf Wallstreet Online.