| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,065 | 0,090 | 13:05 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 06.01. | TME Pharma auf der Suche nach Partnern | 26 | transkript.de | ||
| 05.01. | Cash-strapped TME Pharma loses partner, prompting eye drug strategy rethink | 8 | FierceBiotech | ||
| 05.01. | TME Pharma NV: TME Pharma provides update on its activities | 394 | GlobeNewswire (Europe) | TME Pharma provides update on its activities Berlin, Germany, January 5, 2025, 08.00 CET - TME Pharma N.V. (Euronext Growth Paris: ALTME), a clinical-stage biotechnology company specializing in the... ► Artikel lesen | |
| 17.11.25 | TME Pharma announces receipt of agreements in principle to extend certain bond agreements | 710 | GlobeNewswire (Europe) | TME Pharma announces receipt of agreements in principle to extend certain bond agreements Berlin, Germany, November 17, 2025, 08.00am CET - TME Pharma N.V. (Euronext Growth Paris: ALTME), a clinical-stage... ► Artikel lesen | |
| 05.11.25 | TME Pharma advances its new investment strategy and signs LOI with German resources company | 236 | GlobeNewswire (Europe) | TME Pharma advances its new investment strategy and signs LOI with German resources company A further step toward investment diversification into potential cash-flow-generating assets A potential... ► Artikel lesen | |
| 22.09.25 | TME Pharma N.V.: TME Pharma Announces Date of Publication of Half Year Results and CEO Share Purchase | 494 | Business Wire | Regulatory News:
TME Pharma N.V. (Euronext Growth Paris: ALTME), a clinical-stage biotechnology company specializing in the development of novel therapies for cancer and eye diseases, announces... ► Artikel lesen | |
| 01.09.25 | TME Pharma N.V.: TME Pharma Completes August Financing and Publishes Cash Position | 486 | Business Wire | TME Pharma has completed the fund raise announced last August 25th, receiving €500k in cash on August 28th in addition to the €1.7M raised end of May Cash position €2.35M at end of August... ► Artikel lesen | |
| 25.08.25 | TME Pharma N.V.: TME Pharma to raise €500k through new bond issue and receives new notice of shareholding | 431 | Business Wire | TME Pharma is raising additional funds to accelerate the Company's fundamental activities and to increase its budget for its treasury investment strategy TME Pharma has secured binding... ► Artikel lesen | |
| TME PHARMA Aktie jetzt für 0€ handeln | |||||
| 29.07.25 | TME Pharma N.V.: TME Pharma Provides Update About Additional Strategy | 675 | Business Wire | Regulatory News:
TME Pharma N.V. (Euronext Growth Paris: ALTME), a clinical-stage biotechnology company specializing in the development of novel therapies for cancer and eye diseases, is pleased... ► Artikel lesen | |
| 08.07.25 | TME Pharma N.V.: TME Pharma: Termination of the Liquidity Contract | 587 | Business Wire | Regulatory News:
TME Pharma N.V. (Euronext Growth Paris: ALTME), a clinical stage biotechnology company focused on developing novel therapies for treatment of cancer and eye diseases, announced... ► Artikel lesen | |
| 30.06.25 | TME Pharma N.V.: TME Pharma Announces New Treasury Investment Policy | 609 | Business Wire | Part of treasury funds can now be allocated to cryptocurrency-linked investments If successful, the company may consider expanding cryptocurrency strategies beyond treasury management to... ► Artikel lesen | |
| 27.06.25 | TME Pharma N.V.: TME Pharma Provides Results of Final Exercise of Warrants Z | 591 | Business Wire | 1,948 Warrants Z were exercised resulting in the issuance of 2,435 new ordinary shares
Regulatory News:
TME Pharma N.V. (Euronext Growth Paris: ALTME), a clinical-stage biotechnology company... ► Artikel lesen | |
| 25.06.25 | TME Pharma N.V.: TME Pharma Appoints Diede van den Ouden as New CEO at the 2025 Annual General Meeting of Shareholders | 682 | Business Wire | Regulatory News:
TME Pharma N.V. (Euronext Growth Paris: ALTME), a clinical-stage biotechnology company focused on developing novel therapies for treatment of cancer by targeting the tumor microenvironment... ► Artikel lesen | |
| 18.06.25 | TME Pharma N.V.: TME Pharma Announces Successful Next Step in Its NOX-E36 Ophthalmology Strategy With Signature of Option Framework Agreement With Singapore Eye Research Institute (SERI) | 511 | Business Wire | Option framework agreement with SERI establishes ownership- and revenue-sharing model for all NOX-E36 ophthalmic disease rights TME Pharma to lead commercial discussions for potential spin-out... ► Artikel lesen | |
| 27.05.25 | TME Pharma N.V.: TME Pharma Provides Additional Details on the Debt and Warrant Agreements Announced on 21 May That Extend Its Financial Visibility by 12 Months | 647 | Business Wire | The Company is hereby clarifying and providing additional information on the financing announced on May 21, 2025 TME Pharma has secured private binding commitments from professional European... ► Artikel lesen | |
| 26.05.25 | TME Pharma N.V.: TME Pharma Announces Convocation of the 2025 Annual General Meeting of Shareholders | 1.107 | Business Wire | Regulatory News:
TME Pharma N.V. (Euronext Growth Paris: ALTME),a clinical-stage biotechnology company focused on developing novel therapies for treatment of cancer by targeting the tumor microenvironment... ► Artikel lesen | |
| 21.05.25 | TME Pharma N.V.: TME Pharma Extends Its Financial Visibility by 12 Months | 782 | Business Wire | TME Pharma secures binding commitments for €1.7M proceeds from regular bonds repayable in cash during 12-month maturity period The transaction extends financial visibility into May 2026... ► Artikel lesen | |
| 05.05.25 | TME Pharma N.V.: TME Pharma Announces That Nominated CEO Plans to Increase His Shareholding | 548 | Business Wire | Diede van den Ouden has disclosed plans for initial share purchases to become a more significant shareholder in TME Pharma prior to his nomination for appointment as CEO at the upcoming AGM in... ► Artikel lesen | |
| 05.05.25 | TME Pharma N.V.: TME Pharma Announces New Strategy | 1.462 | Business Wire | Diede van den Ouden, an experienced CEO with successful background in small-cap turnarounds, to be nominated for appointment as CEO at upcoming AGM in June 2025 Operational costs to be... ► Artikel lesen | |
| 25.04.25 | TME Pharma N.V.: TME Pharma Publishes 2024 Financial Results and Provides Operating Update | 590 | Business Wire | Total €7.6 million raised through multiple transactions All convertible debt instruments removed from balance sheet during 2024 Commitment to lean cost structure while focusing on... ► Artikel lesen |
1 2 Weiter >>
24 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | Ionos, Kontron, Lanxess, Qiagen, Redcare Pharmacy, Siltronic, TeamViewer: Neue Aktien-Positionen der Short-Seller | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend |
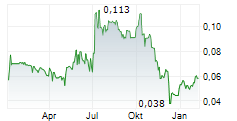
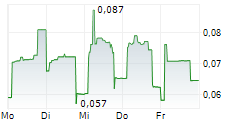
TME PHARMA NV gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 0,065 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu TME PHARMA NV finden Sie auf Wallstreet Online.